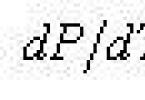Ph.D. O.V. Mosin
MOLECULAR PHYSICS OF WATER IN ITS THREE STATES OF AGGREGATE
Water, hydrogen oxide, H 2 0, the simplest chemical compound of hydrogen and oxygen that is stable under normal conditions (11.19% hydrogen and 88.81% oxygen by mass). Water is a colorless, odorless, tasteless liquid (in thick layers it has a bluish color), which plays a vital role in the geological history of the Earth and the emergence of life, in the formation of the physical and chemical environment, climate and weather on our planet. Water is an essential component of almost all technological processes - both agricultural and industrial production.
Water is part of all living organisms, and in general they contain only half as much water as all the rivers on Earth. In living organisms, the amount of water, excluding seeds and spores, varies between 60 and 99.7% by weight. According to the French biologist E. Dubois-Reymond, a living organism is l "eau animée (animate water). All the waters of the Earth constantly interact with each other, as well as with the atmosphere, lithosphere and biosphere.
The globe contains about 16 billion km3 of water, which is 0.25% of the mass of our entire planet. Of this amount, the Earth's hydrosphere (oceans, seas, lakes, rivers, glaciers and groundwater) accounts for 1.386 billion km3. Fresh surface water (lakes and rivers) is only 0.2 million km3, and atmospheric water vapor is 13 thousand km3.
The total mass of snow and ice distributed over the Earth's surface reaches approximately 2.5-3.0 x 1016 tons, which is only 0.0004% of the mass of our entire planet. However, such an amount is enough to cover the entire surface of the Earth with a 53-meter layer, and if all this mass suddenly melted, turning into water, then the level of the World Ocean would rise by about 64 meters compared to the current level.
The waters of the Earth penetrate it, starting from the highest heights of the stratosphere down to the enormous depths of the earth's crust, reaching the mantle, and form a continuous shell of the planet - the hydrosphere, which includes all water in a liquid, solid, gaseous, chemically and biologically connected state.
Hydrosphere - the watery shell of the Earth, including oceans, seas, lakes, reservoirs, rivers, groundwater, soil moisture, is about 1.4-1.5 billion km 3, with land water accounting for only about 90 million km 3. Of these, groundwater makes up 60, glaciers 29, lakes 0.75, soil moisture 0.075, rivers 0.0012 million km 3.
The hydrosphere has played and continues to play a fundamental role in the geological history of the Earth, in the formation of the physical and chemical environment, climate and weather, and in the emergence of life on our planet. It developed together and in close interaction with the lithosphere, atmosphere, and then living nature.
In the atmosphere water is in the form of steam, fog and clouds, raindrops and snow crystals (about 13-15 thousand km 3 in total). About 10% of the land surface is permanently occupied by glaciers. In the north and northeast of the USSR, in Alaska and the north of Canada - with a total area of about 16 million km 2, a subsoil layer of ice is always preserved (only about 0.5 million km 3.
In the earth's crust - lithosphere contains, according to various estimates, from 1 to 1.3 billion km3 of water, which is close to its content in the hydrosphere. In the earth's crust, significant amounts of water are in a bound state, being part of some minerals and rocks (gypsum, hydrated forms of silica, hydrosilicates, etc.). Huge amounts of water (13-15 billion km 3) are concentrated in the deeper depths of the Earth's mantle. The release of water released from the mantle during the heating of the Earth in the early stages of its formation gave rise, according to modern views, to the hydrosphere. The annual supply of water from the mantle and magma chambers is about 1 km 3.
There is evidence that water, at least partially, has a “cosmic” origin: protons that came into the upper atmosphere from the Sun, capturing electrons, turn into hydrogen atoms, which, combining with oxygen atoms, give H 2 O.
Water is found in natural conditions in three states: solid - in the form of ice and snow, liquid - in the form of water itself, gaseous - in the form of water vapor. These states of water are called aggregate states, or solid, liquid and vapor phases, respectively. The transition of water from one phase to another is caused by changes in its temperature and pressure. In Fig. Figure 1 shows a diagram of the states of aggregation of water depending on temperature t and pressure P. From Fig. 1. it is clear that in region I water is found only in solid form, in region II - only in liquid form, in region III - only in the form of water vapor. Along the AC curve it is in a state of equilibrium between solid and liquid phases (ice melting and water crystallization); along the AB curve - in a state of equilibrium between the liquid and gaseous phases (evaporation of water and condensation of steam); along the AD curve - in equilibrium between the solid and gaseous phases (sublimation of water vapor and sublimation of ice).
Rice. 1. Diagram of aggregate states of water in the region of triple point A. I - ice. II - water. III - water vapor.
The equilibrium of the phases according to Fig. 1 along the curves AB, AC and AD should be understood as dynamic equilibrium, i.e. along these curves the number of newly formed molecules of one phase is strictly equal to the number of newly formed molecules of the other phase. If, for example, we gradually cool water at any pressure, then in the limit we will find ourselves on the AC curve, where water will be observed at the corresponding temperature and pressure. If we gradually heat ice at different pressures, we will find ourselves on the same AC equilibrium curve, but on the ice side. Similarly, we will have water and water vapor, depending on which side we approach the AB curve.
All three curves of the state of aggregation - AC (curve of the dependence of the melting temperature of ice on pressure), AB (curve of the dependence of the boiling point of water on pressure), AD (curve of the dependence of the vapor pressure of the solid phase on temperature) - intersect at one point A, called the triple point . According to modern research, the values of saturation vapor pressure and temperature at this point are respectively equal: P = 610.6 Pa (or 6.1 hPa = 4.58 mm Hg), t = 0.01°C (or T = 273.16 K). In addition to the triple point, the AB curve passes through two more characteristic points - the point corresponding to the boiling of water at normal air pressure with coordinates P = 1.013 10 5 Pa and t = 100°C, and the point with coordinates P = 2.211 10 7 Pa and t cr = 374.2°C, corresponding to the critical temperature - the temperature only below which water vapor can be converted into a liquid state by compression.
Curves AC, AB, AD related to the processes of transition of a substance from one phase to another are described by the Clapeyron-Clausius equation:
![]()
where T is the absolute temperature corresponding for each curve, respectively, to the temperature of evaporation, melting, sublimation, etc.; L - specific heat of evaporation, melting, sublimation, respectively; V 2 – V 1 - the difference in specific volumes, respectively, when moving from water to ice, from water vapor to water, from water vapor to ice.
Direct experience shows that natural land waters at normal atmospheric pressure supercool (curve AF) to certain negative temperatures without crystallizing. Thus, water has the property of being supercooled, i.e. accept temperatures below the melting point of ice. The supercooled state of water is a metastable (unstable) state in which the transition of the liquid phase into the solid phase, which began at any point, continues continuously until the supercooling is eliminated or until all the liquid turns into a solid. The ability of water to reach temperatures below the melting point of ice was first discovered by Fahrenheit back in 1724.
 Thus, ice crystals can only form in supercooled water. The transition of supercooled water into a solid state - ice, occurs only if there are centers (nuclei) of crystallization in it, which can be suspended sediment particles in the water, ice or snow crystals entering the water from the atmosphere, ice crystals formed in supercooled water as a result of its turbulent translational movement, particles of other substances present in the water column.
Thus, ice crystals can only form in supercooled water. The transition of supercooled water into a solid state - ice, occurs only if there are centers (nuclei) of crystallization in it, which can be suspended sediment particles in the water, ice or snow crystals entering the water from the atmosphere, ice crystals formed in supercooled water as a result of its turbulent translational movement, particles of other substances present in the water column.
Rice. 2. Phase diagram of water. Ih, II - IX - forms of ice; 1 - 8 - triple points.
Supercooling of water is a thermodynamic state in which the temperature of water is below its crystallization temperature. This condition occurs as a result of a decrease in water temperature or an increase in its crystallization temperature. The water temperature can be lowered by removing heat, which is most often found in nature, or by mixing it with salt water, such as sea water. The crystallization temperature can be increased by lowering the pressure.
In laboratory conditions, with high pressure and intensive cooling, distilled water can be supercooled to a temperature of the order of - 30, and drops - 50 ° C. The rate of its crystallization also depends on the depth of supercooling of water.
Thus, the diagram of states of aggregation of water is the solid line AD in Fig. 1 - should be considered as relating to very low thermal loads, when the effect of time on phase transformation is small. At high thermal loads, the process of phase transformations will occur according to the dashed curve AF.
The melting temperature of ice (AC curve) depends very little on pressure. Almost the AC curve is parallel to the horizontal axis: when the pressure changes from 610.6 to 1.013·10 5 Pa, the melting point decreases only from 0.01 to 0°C. However, this temperature decreases with increasing pressure only to a certain value, then it increases and at very high pressure reaches a value of the order of 450°C (Fig. 1.2). As follows from Fig. 1.2, at high pressure ice can also be at a positive temperature. There are up to ten different forms of ice. The form of ice Ih, which is characterized by a decrease in melting point with increasing pressure, corresponds to ordinary ice formed due to the freezing of water under normal conditions. The coordinates of the triple points of various forms of ice, indicated in Fig. 1.2 by Arabic numerals 1-8, are given in Table. 1.1. The structure and physical properties of all forms of ice are significantly different from Ih ice.
A solid (ice), like a liquid, evaporates over a wide range of temperatures and directly transforms into a gaseous state (sublimation), bypassing the liquid phase - AD curve. The reverse process, i.e., the transition of a gaseous form directly into a solid form (sublimation), is carried out, also bypassing the liquid phase. Sublimation and sublimation of ice and snow play a large role in nature.
Structure of a water molecule
 Water is a complex substance, the main structural unit of which is the H 2 O molecule, consisting of two hydrogen atoms and one oxygen atom. Several dozen schemes for the possible mutual arrangement of H and O atoms in the H 2 O molecule have been proposed over the entire period of its study; The currently generally accepted scheme is shown in Fig. 3.
Water is a complex substance, the main structural unit of which is the H 2 O molecule, consisting of two hydrogen atoms and one oxygen atom. Several dozen schemes for the possible mutual arrangement of H and O atoms in the H 2 O molecule have been proposed over the entire period of its study; The currently generally accepted scheme is shown in Fig. 3.
Rice. 3. Scheme of the structure of a water molecule: molecular geometry and electron orbits
The total kinetic energy of a triatomic molecule like H 2 O can be described by the following expression:
![]()
where and are the speeds of translational and rotational motion of the molecule, respectively; I x , I y , I z - moments of inertia of the molecule relative to the corresponding axes of rotation; m is the mass of the molecule.
From this equation it is clear that the total energy of a triatomic molecule like H 2 O consists of six parts corresponding to six degrees of freedom: three translational and three rotational.
It is known from the physics course that each of these degrees of freedom in thermal equilibrium accounts for the same amount of energy equal to 1/2 kT, where k=R m /N A = 1.3807·10 -23 J/K is Boltzmann’s constant; T-absolute temperature; N A = 6.0220·10 23 mol -1 - Avogadro's number; kN A =R m = 8.3144 J/(mol K) - universal gas constant. Then the total kinetic energy of such a molecule is equal to:
![]()
The total kinetic energy of the molecules contained in a gram molecule of any gas (vapor) will be:

The total kinetic energy W is related to the specific heat capacity cv at constant volume by the formula:

Calculating the specific heat capacity of water using this formula for water vapor gives a value of 25 J/(mol K). According to experimental data, for water vapor cv = 27.8 J/(mol K), i.e., close to the calculated value.
Studying the water molecule using spectrographic studies has made it possible to establish that it has the structure of a sort of isosceles triangle: at the top of this triangle there is an oxygen atom, and at its base there are two hydrogen atoms. The apex angle is 104°27, and the side length is 0.096 nm. These parameters refer to the hypothetical equilibrium state of the molecule without its vibrations and rotations.
The relative molecular mass of H 2 O depends on the relative atomic mass of its components and has different values, since oxygen and hydrogen have isotopes.
Oxygen has six isotopes: 14 O, 15 O, 16 O, 17 O, 18 O, 19 O, of which only three are stable, and hydrogen has three: 1 H (protium), 2 H (deuterium), 3 H (tritium) . Some of the isotopes are radioactive, have a short half-life and are present in water in small quantities, while others are obtained only artificially and are not found in nature.
Thus, taking into account the isotopes of oxygen and hydrogen, it is possible to compose from them several types of the H 2 O molecule with different relative molecular masses. Of these, the most common are 1 H 2 16 O molecules with a relative molecular weight of 18 (ordinary water) and 2 H 2 16 O molecules with a relative molecular weight of 20. The latter molecules form so-called heavy water. Heavy water differs significantly in its physical properties from ordinary water.
Molecular-kinetic theory of matter and water
The structure of water in its three states of aggregation cannot yet be considered finally established. There are a number of hypotheses explaining the structure of steam, water and ice.
These hypotheses are to a greater or lesser extent based on the molecular kinetic theory of the structure of matter, the foundations of which were laid by M.V. Lomonosov. In turn, the molecular kinetic theory is based on the principles of classical mechanics, in which molecules (atoms) are considered as balls of regular shape, electrically neutral, ideally elastic. Such molecules are subject only to mechanical collisions and do not experience any electrical interaction forces. For these reasons, the use of molecular kinetic theory can only explain the structure of matter to a first approximation.
Gas - in our case water vapor - according to molecular kinetic theory, is a collection of molecules. The distance between them is many times greater than the size of the molecules themselves. Gas molecules are in continuous random motion, running a path between the walls of the vessels in which the gas is contained, and colliding with each other along this path. Collisions between molecules occur without loss of mechanical energy; they are considered as collisions of perfectly elastic balls. The impacts of molecules on the walls of the container limiting them determine the pressure of the gas on these walls. The speed of movement of molecules increases with increasing temperature and decreases with its fall.
When the gas temperature, decreasing from higher values, approaches the boiling point of the liquid (for water 100 ° C at normal pressure), the speed of the molecules decreases, and upon collision, the attractive forces between them become greater than the elastic repulsion forces upon impact and therefore the gas condenses into a liquid .
When artificially liquefying gas, its temperature must be below the so-called critical temperature, which also corresponds to the critical pressure (clause 1.1). At temperatures above critical, gas (steam) cannot be converted into liquid by any pressure.
The value of RT cr / (P cr V cr) for all gases, including water vapor, should be equal to 8/3 = 2.667 (here R is the gas constant; T cr, P cr, V cr are the critical temperatures, respectively, pressure, volume). However, for water vapor it is 4.46. This is explained by the fact that the vapor contains not only single molecules, but also their associations.
A liquid, unlike a gas, is a collection of molecules located so close to each other that forces of mutual attraction appear between them. Therefore, liquid molecules do not fly apart in different directions, like gas molecules, but only oscillate around their equilibrium position. At the same time, since the structure of the liquid is not completely dense, there are free places in it - “holes”, as a result of which, according to the theory of Ya.I. Frenkel, some molecules with greater energy break out of their “settled” place and move abruptly into a neighboring “hole” located at a distance approximately equal to the size of the molecule itself. Thus, in a liquid, molecules move relatively rarely from place to place, and most of the time they are in a “settled” state, only undergoing oscillatory movements. This, in particular, explains the weak diffusion in liquids compared to its high speed in gases. When a liquid is heated, the energy of its molecules increases and the speed of their vibration increases. At a temperature of 100°C and normal atmospheric pressure, water breaks down into individual H2O molecules, the speed of which is already able to overcome the mutual attraction of the molecules, and the water turns into steam.
When cooling a liquid (water), the reverse process occurs. The speed of vibrational motion of molecules decreases, the structure of the liquid becomes stronger, and the liquid turns into a crystalline (solid) state - ice. There are two types of solids: crystalline and amorphous. The main feature of crystalline bodies is the anisotropy of their properties in various directions: thermal expansion, strength, optical and electrical properties, etc. Amorphous bodies are isotropic, that is, they have the same properties in all directions. Ice is a crystalline solid.
In a solid, unlike gases and liquids, each atom or molecule vibrates only about its equilibrium position, but does not move. There are no “holes” in a solid into which individual molecules can pass. Therefore, there is no diffusion in solids. The atoms that make up molecules form a strong crystal lattice, the immutability of which is due to molecular forces. When the temperature of a solid approaches its melting point, its crystal lattice is destroyed and it turns into a liquid state. In contrast to the crystallization of liquids, the melting of solids occurs relatively slowly, without a pronounced jump.
Crystallization of most liquids occurs with a decrease in volume, and the melting of solids is accompanied by an increase in volume. The exceptions are water, antimony, paraffin and some other substances whose solid phase is less dense than the liquid.
Structure of water in its three states of aggregation
The problem of assessing the structure of water still remains one of the most difficult. Let us briefly consider two generalized hypotheses about the structure of water that received the greatest recognition, one in the initial period of development of the doctrine of the structure of water, the other at the present time.
According to the hypothesis proposed by Whiting (1883) and which currently has various interpretations, the main building unit of water vapor is the H 2 O molecule, called a hydrol, or monohydrol. The basic building unit of water is the double water molecule (H 2 O) 2 -dihydrol; ice consists of triple molecules (H 2 O) 3 - trihydrol. The so-called hydrol theory of the structure of water is based on these ideas.
Water vapor, according to this theory, consists of a collection of the simplest monohydrol molecules and their associations, as well as a small amount of dihydrol molecules.
Liquid water is a mixture of monohydrol, dihydrol and trihydrol molecules. The ratio of the number of these molecules in water is different and depends on temperature. According to this hypothesis, the ratio of the number of water molecules explains one of its main anomalies - the highest density of water at 4°C.
Since the water molecule is asymmetrical, the centers of gravity of its positive and negative charges do not coincide. Molecules have two poles - positive and negative, creating, like a magnet, molecular force fields. Such molecules are called polar, or dipoles, and the quantitative characteristic of polarity is determined by the electric moment of the dipole, expressed by the product of the distance l between the electrical centers of gravity of the positive and negative charges of the molecule by the charge e in absolute electrostatic units:
For water, the dipole moment is very high: p = 6.13·10 -29 C m. The polarity of monohydrol molecules explains the formation of dihydrol and trihydrol. At the same time, since the intrinsic speeds of molecules increase with increasing temperature, this can explain the gradual decomposition of a trihydrol into a dihydrol and then into a monohydrol, respectively, when ice melts, water is heated and boils.
Another hypothesis of the structure of water, developed in the 20th century (models by O.Ya. Samoilov, J. Pople, G.N. Zatsepina, etc.), is based on the idea that ice, water and water vapor consist of H 2 O molecules united into groups using so-called hydrogen bonds (J. Bernal and R. Fowler, 1933). These bonds arise from the interaction of the hydrogen atoms of one molecule with the oxygen atom of a neighboring molecule (with a highly electronegative element). This feature of hydrogen exchange in a water molecule is due to the fact that, giving up its only electron to form a covalent bond with oxygen, it remains in the form of a nucleus, almost devoid of an electron shell. Therefore, the hydrogen atom does not experience repulsion from the electron shell of oxygen of the neighboring water molecule, but, on the contrary, is attracted by it and can interact with it. According to this hypothesis, it can be assumed that the forces forming a hydrogen bond are purely electrostatic. However, according to the molecular orbital method, hydrogen bonding is formed by dispersion forces, covalent bonding, and electrostatic interaction.
Table 1 shows the molecular composition of water, ice and water vapor according to various literature sources.
Table 1.1
Molecular composition of ice, water and water vapor, %

Thus, as a result of the interaction of hydrogen atoms of one water molecule with the negative charges of oxygen of another molecule, four hydrogen bonds are formed for each water molecule. In this case, molecules, as a rule, are combined into groups - associates: each molecule is surrounded by four others (Fig. 4). Such a dense packing of molecules is characteristic of water in the frozen state (ice Ih) and leads to an open crystal structure belonging to hexagonal symmetry. With this structure, “voids - channels” are formed between fixed molecules, so the density of ice is less than the density of water.
 Increasing the temperature of ice until it melts and above leads to the breaking of hydrogen bonds. In the liquid state of water, even ordinary thermal movements of molecules are sufficient to destroy these bonds.
Increasing the temperature of ice until it melts and above leads to the breaking of hydrogen bonds. In the liquid state of water, even ordinary thermal movements of molecules are sufficient to destroy these bonds.
Rice. 4. Scheme of interaction of water molecules. 1 - oxygen, 2 - hydrogen, 3 - chemical bond, 4 - hydrogen bond.
When the water temperature increases to 4°C, the ordering of the arrangement of molecules according to the crystalline type with a characteristic structure for ice is preserved to some extent. The above-mentioned voids in this structure are filled with released water molecules. As a result, the density of the liquid increases to its maximum at a temperature of 3.98°C. A further increase in temperature leads to the distortion and breaking of hydrogen bonds, and, consequently, the destruction of groups of molecules, down to individual molecules, which is typical for steam.
So what are the mysterious, unusual properties of the familiar liquid water? First of all, the fact is that almost all the properties of water are anomalous, and many of them do not obey the logic of those laws of physics that govern other substances.
When water molecules condense, they form a liquid substance of amazing complexity. This is primarily due to the fact that water molecules have the unique property of combining into clusters (groups) (H 2 O)x. A cluster is usually understood as a group of atoms or molecules united by physical interaction into a single ensemble, but retaining individual behavior within it. The possibilities for direct observation of clusters are limited, and therefore experimenters compensate for instrumental shortcomings with intuition and theoretical constructs.
At room temperature, the degree of association X for water, according to modern data, is from 3 to 6. This means that the formula of water is not just H 2 O, but an average between H 6 O 3 and H 12 O 6. In other words, water is a complex liquid “made up” of repeating groups containing three to six single molecules. As a result, water has abnormal freezing and boiling point values compared to its homologues. If water obeyed general rules, it should freeze at a temperature of about -100 o C and boil at a temperature of about +10 o C.
If water remained in the form of H 6 O 3, H 8 O 4 or H 12 O 6 during evaporation, then water vapor would be much heavier than air, in which nitrogen and oxygen molecules dominate. In this case, the surface of the entire Earth would be covered with an eternal layer of fog. It is almost impossible to imagine life on such a planet.
People are very lucky: water clusters disintegrate during evaporation, and the water turns practically into a simple gas with the chemical formula H 2 O (the small amount of H 4 O 2 dimers recently discovered in steam does not make a difference). The density of gaseous water is less than the density of air, and therefore, water is capable of saturating the earth’s atmosphere with its molecules, creating comfortable weather conditions for humans.
There are no other substances on Earth that are endowed with the ability to be a liquid at the temperatures of human existence and at the same time form a gas that is not only lighter than air, but also capable of returning to its surface in the form of precipitation.
Ph.D. O.V. Mosin
Introduction
1. The structure of water molecules
2. Structure of water in its three states of aggregation
3. Types of water
4. Anomalous properties of water
5. Phase transformations and state diagram of water
6. Models of the structure of water and ice
7. Aggregate types of ice
Conclusion
Bibliography
Introduction
Water is the most important substance on Earth without which no living organism can exist and no biological, chemical reactions or technological processes can occur.
Water (hydrogen oxide) is an odorless, tasteless and colorless liquid (bluish in thick layers); H 2 O, mol. m. 18.016, the simplest stable connection. hydrogen with oxygen.
Water is one of the most common substances in nature. It covers about 3/4 of the entire earth's surface, forming the basis of oceans, seas, lakes, rivers, pound waters and swamps. A large amount of water is also found in the atmosphere. Plants and living organisms contain 50-96% water.
Water molecules have been discovered in interstellar space. Water is part of comets, most planets in the solar system and their satellites. The amount of water on the Earth's surface is estimated at 1.39 * 10 18 tons, most of it is contained in the seas and oceans. The amount of fresh water available for use in rivers, lakes, swamps and reservoirs is 2 * 10 4 tons. The mass of glaciers in Antarctica, Antarctica and high mountain regions is 2.4 * 10 16 tons (the total mass of snow and ice distributed over the Earth's surface reaches approximately 2.5-3.010 16 tons, which is only 0.0004% of the mass of our entire planet.However, such an amount is enough to cover the entire surface of the Earth with a 53-meter layer, and if all this mass suddenly melted, having turned into water, the level of the World Ocean would have risen by about 64 meters compared to the current one.), there is approximately the same amount of groundwater, and only a small part of it is fresh. In the atmosphere there is approx. 1.3*10 13 tons of water. Water is part of many minerals and rocks (clay, gypsum, etc.), is present in the soil, and is an essential component of all living organisms.
Density of H 2 O = 1 g/cm3 (at 3.98 degrees), t pl. = 0 degrees, and t kip = 100 degrees. The heat capacity of water is 4.18 J/(g/K) Mr (H 2 O) = 18 and corresponds to its simplest formula. However, the molecular weight of liquid water, determined by studying its solutions in other solvents, turns out to be higher. This indicates that in liquid water there is an association of molecules, i.e., they are combined into more complex aggregates. Water is the only substance in nature that, under terrestrial conditions, exists in all three states of aggregation: Much water is in a gaseous state in the form of vapor in the atmosphere; it lies in the form of huge masses of snow and ice all year round on the tops of high mountains and in polar countries. In the bowels of the earth there is also water that saturates the soil and rocks
Climate depends on water. Geophysicists claim that the Earth would have cooled long ago and turned into a lifeless piece of stone if it were not for water. It has a very high heat capacity. When heated, it absorbs heat; cooling down, he gives it away. Earth's water both absorbs and returns a lot of heat and thereby “evens out” the climate. And what protects the Earth from the cosmic cold are those water molecules that are scattered in the atmosphere - in clouds and in the form of vapor... You cannot do without water - this is the most important substance on Earth.
Water is a familiar and unusual substance. Famous Soviet scientist
Academician I.V. Petryanov called his popular science book about water “the most extraordinary substance in the world.” And “Entertaining Physiology,” written by Doctor of Biological Sciences B.F. Sergeev, begins with a chapter about water - “The Substance that Created Our Planet.”
1. Structure of a water molecule
Of all common liquids, water is the most universal solvent, the liquid with the maximum values of surface tension, dielectric constant, heat of vaporization and the highest (after ammonia) heat of fusion. Unlike most substances, water expands when it freezes at low pressure.
These specific properties of water are associated with the special structure of its molecule. The chemical formula of water, H 2 0, is deceptively simple. In a water molecule, the nuclei of hydrogen atoms are located asymmetrically with respect to the nucleus of the oxygen atom and electrons. If the oxygen atom is at the center of the tetrahedron, the centers of mass of the two hydrogen atoms will be in the corners of the tetrahedron, and the centers of charge of the two pairs of electrons will occupy the other two corners (Fig. 1.1). Thus, four electrons are located at the greatest possible distance both from the nucleus of the oxygen atom and from the nuclei of the hydrogen atoms, at which they are still attracted by the nucleus of the oxygen atom. The other six electrons of the water molecule are arranged as follows: four electrons are in a position that provides a chemical bond between the nuclei of the oxygen and hydrogen atoms, and the other two are located near the nucleus of the oxygen atom.
The asymmetric arrangement of the atoms of a water molecule causes an uneven distribution of electrical charges in it, which makes the water molecule polar. This structure of the water molecule causes the attraction of water molecules to each other as a result of the formation of hydrogen bonds between them. The arrangement of hydrogen and oxygen atoms inside the formed aggregates of water molecules is similar to the arrangement of silicon and oxygen atoms in quartz. This applies to ice and, to a lesser extent, to liquid water, whose molecular aggregates are always in the stage of redistribution. When water cools, its molecules group into aggregates, which gradually increase in size and become more stable as the temperature approaches 4° C, when the water reaches its maximum density. At this temperature, water does not yet have a rigid structure and, along with long chains of its molecules, there are a large number of individual water molecules. With further cooling, the chains of water molecules grow due to the addition of free molecules to them, as a result of which the density of water decreases. When water turns into ice, all its molecules enter a more or less rigid structure in the form of open chains that form crystals.
Fig. 1.1 Structure of a water molecule
Mutual penetration of hydrogen and oxygen atoms. The nuclei of two hydrogen atoms and two pairs of electrons are located in the corners of the tetrahedron: in the center is the nucleus of an oxygen atom.
The high values of surface tension and heat of vaporization of water are explained by the fact that a relatively large expenditure of energy is required to separate a water molecule from a group of molecules. The tendency of water molecules to form hydrogen bonds and their polarity explain the unusually high solubility of water. Some compounds, such as sugars and alcohols, are held in solution by hydrogen bonds. Compounds that are highly ionized, such as sodium chloride, are held in solution because ions with opposite charges are neutralized by groups of oriented water molecules.
Another feature of the water molecule is that both hydrogen and oxygen atoms can have different masses with the same nuclear charge. Varieties of a chemical element with different atomic weights are called isotopes of that element. A water molecule is usually formed by hydrogen with atomic weight 1 (H 1) and oxygen with atomic weight 16 (O 16). More than 99% of water atoms belong to these isotopes. In addition, the following isotopes exist: H 2, H 3, O 14, O 15, O 17, O 18, O 19. Many of them accumulate in water as a result of its partial evaporation and due to their large mass. The isotopes H 3, O 14, O 15, O 19 are radioactive. The most common of them is tritium H 3, formed in the upper layers of the atmosphere under the influence of cosmic rays. This isotope has also accumulated as a result of nuclear explosions over the past few years. Based on these and other facts about isotopes, analysis of the isotopic composition of water can partially reveal the history of some natural waters. Thus, the content of heavy isotopes in surface waters indicates long-term evaporation of water, which occurs, for example, in the Dead Sea, the Great Salt Lake and other closed reservoirs. Elevated levels of tritium in groundwater could mean that these waters are of meteoric origin with a high circulation rate, because the half-life of this isotope is only 12.4 years. Unfortunately, isotope analysis is too expensive and for this reason cannot be widely used in studies of natural waters.
The water molecule H2O is built in the form of a triangle: the angle between the two oxygen-hydrogen bonds is 104 degrees. But since both hydrogen atoms are located on the same side of the oxygen, the electrical charges in it are dispersed. The water molecule is polar, which is the reason for the special interaction between its different molecules.
The hydrogen atoms in the H2O molecule, having a positive partial charge, interact with the electrons of the oxygen atoms of neighboring molecules. This chemical bond is called a hydrogen bond. It combines H 2 O molecules into unique polymers of a spatial structure; the plane in which the hydrogen bonds are located is perpendicular to the plane of the atoms of the same H 2 O molecule. The interaction between water molecules primarily explains the abnormally high temperatures of its melting and boiling. Additional energy must be supplied to loosen and then destroy hydrogen bonds. And this energy is very significant. This is why the heat capacity of water is so high.
Like most substances, water is made up of molecules, and the latter are made up of atoms.
The idea of ancient philosophers that everything in nature is formed by four elements (elements): earth, air, fire and water, existed until the Middle Ages. In 1781, G. Cavendish reported that he had obtained water by burning hydrogen, but did not fully appreciate the importance of his discovery. Later (1783)A. Lavoisier proved that water is not an element at all, but a compound of hydrogen and oxygen. J. Berzelius and P. Dulong (1819), as well as J. Dumas and J. Stas (1842), established the weight composition of water by passing hydrogen through copper oxide, taken in a strictly defined amount, and weighing the resulting copper and water. From these data, they determined the H:O ratio for water. In addition, in the 1820s, J. Gay-Lussac measured the volumes of gaseous hydrogen and oxygen, which, when interacting, gave water: they correlated with each other as 2: 1, which, as we now know, corresponds to the formula H 2 O. Prevalence. Water covers 3/4 of the Earth's surface. The human body consists of about 70% water, the egg is 74%, and some vegetables are almost entirely water. So, in watermelon it is 92%, in ripe tomatoes - 95%.Water in natural reservoirs is never homogeneous in composition: it passes through rocks, comes into contact with soil and air, and therefore contains dissolved gases and minerals. Distilled water is purer.
Sea water . The composition of seawater varies in different regions and depends on the influx of fresh water, evaporation rate, precipitation, melting of icebergs, etc.see also OCEAN.Mineral water. Mineral water is formed when ordinary water seeps through rocks containing compounds of iron, lithium, sulfur and other elements.Soft and hard water. Hard water contains large quantities of calcium and magnesium salts. They dissolve in water when flowing through rocks composed of gypsum (C aSO 4 ), limestone (CaCO 3 ) or dolomite (carbonates Mg and Ca). Soft water contains little of these salts. If water contains calcium sulfate, it is said to have permanent (non-carbonate) hardness. It can be softened by adding sodium carbonate; this will cause calcium to precipitate as carbonate, leaving sodium sulfate in solution. Sodium salts do not react with soap, and its consumption will be less than in the presence of calcium and magnesium salts.Water with temporary (carbonate) hardness contains calcium and magnesium bicarbonates; it can be softened in several ways: 1) by heating, leading to the decomposition of bicarbonates into insoluble carbonates; 2) adding lime water (calcium hydroxide), as a result of which bicarbonates are converted into insoluble carbonates; 3) using exchange reactions.
Molecular structure. Analysis of data obtained from absorption spectra showed that the three atoms in a water molecule form an isosceles triangle with two hydrogen atoms at the base and oxygen at the apex:The bond angle of HOH is 104.31° , the OH bond length is 0.99Å (1 Å = 10 8 cm), and the distance HH is 1.515 Å . The hydrogen atoms are so deeply embedded in the oxygen atom that the molecule is almost spherical; its radius is 1.38Å . WATER Physical properties. Due to the strong attraction between molecules, water has high melting points (0° C) and boiling (100 ° WITH). A thick layer of water has a blue color, which is determined not only by its physical properties, but also by the presence of suspended particles of impurities. The water of mountain rivers is greenish due to the suspended particles of calcium carbonate it contains. Pure water is a poor conductor of electricity, its specific conductivity is 1.5 H 10 8 Ohm 1 H cm 1 at 0 ° C. The compressibility of water is very low: 43 H 10 6 cm 3 per megabar at 20° C. The density of water is maximum at 4° WITH; this is explained by the properties of the hydrogen bonds of its molecules.Vapor pressure. If you leave water in an open container, it will gradually evaporate and all its molecules will go into the air. At the same time, water located in a tightly sealed vessel evaporates only partially, i.e. at a certain pressure of water vapor, equilibrium is established between water and the air above it. Vapor pressure at equilibrium depends on temperature and is called saturated vapor pressure (or vapor pressure). When the saturated vapor pressure is compared with the external pressure, the water boils. At normal pressure 760 mm Hg. water boils at 100° C, and at an altitude of 2900 m above sea level, atmospheric pressure drops to 525 mm Hg. and the boiling point turns out to be 90° WITH.Evaporation occurs even from the surface of snow and ice, which is why wet laundry dries out in the cold.
The viscosity of water quickly decreases with increasing temperature and at 100
° C turns out to be 8 times less than at 0° C. Chemical properties. Catalytic action. Many chemical reactions occur only in the presence of water. Thus, oxidation with oxygen does not occur in dry gases, metals do not react with chlorine, etc.Hydrates. Many compounds always contain a certain number of water molecules and are therefore called hydrates. The nature of the bonds formed in this case can be different. For example, in copper sulfate pentahydrate, or copper sulfate CuSO 4 H 5H 2 O , four water molecules form coordination bonds with the sulfate ion, which are destroyed at 125° WITH; the fifth water molecule is bound so tightly that it comes off only at a temperature of 250° C. Another stable hydrate sulfuric acid; it exists in two hydrated forms, SO 3 P H 2 O and SO 2 (OH) 2 , between which equilibrium is established. Ions in aqueous solutions are also often hydrated. Yes, N + always exists in the form of hydronium ion H 3 O + or H 5 O 2 + ; lithium ion in the form Li(H2O)6+ etc. Elements as such are rarely found in hydrated form. The exception is bromine and chlorine, which form hydrates Br 2 Ch 10 H 2 O and Cl 2 Ch 6H 2 O. Some common hydrates contain water of crystallization, such as barium chloride BaCl 2 H 2H 2 O , Epsom salt (magnesium sulfate) MgSO 4 H 7H 2 O , baking soda (sodium carbonate) Na 2 CO 3 H 10 H 2 O, Glauber's salt (sodium sulfate) Na 2 SO 4 H 10 H 2 O. Salts can form several hydrates; Thus, copper sulfate exists in the form CuSO 4 H 5H 2 O, CuSO 4 H 3H 2 O and CuSO 4 H H 2 O . If the saturated vapor pressure of the hydrate is greater than atmospheric pressure, the salt will lose water. This process is calledfading (by weathering). The process by which salt absorbs water is calledblurring . Hydrolysis. Hydrolysis is a double decomposition reaction in which one of the reactants is water; phosphorus trichloride PCl 3 easily reacts with water: PCl 3 + 3H 2 O = P (OH) 3 + 3HCl Fats are hydrolyzed in a similar way to form fatty acids and glycerol.Solvation. Water is a polar compound, and therefore readily enters into electrostatic interaction with particles (ions or molecules) of substances dissolved in it. The molecular groups formed as a result of solvation are called solvates. A layer of water molecules bound to the central solvate particle by attractive forces constitutes the solvation shell. The concept of solvation was first introduced in 1891 by I.A. Kablukov.Heavy water. In 1931, G. Urey showed that when liquid hydrogen evaporates, its final fractions turn out to be heavier than ordinary hydrogen due to the content of an isotope that is twice as heavy. This isotope is called deuterium and is represented by the symbol D . In its properties, water containing its heavy isotope instead of ordinary hydrogen differs significantly from ordinary water.In nature, for every 5000 parts by mass N
2 Oh there's one part D2O . This ratio is the same for river, rain, swamp water, groundwater or crystallization water. Heavy water is used as a tracer in the study of physiological processes. Thus, in human urine the ratio between H and D is also equal to 5000:1. If you give the patient water with a high content of D2O , then by consistently measuring the proportion of this water in the urine, you can determine the rate of water excretion from the body. It turned out that about half of the water drunk remains in the body even after 15 days. Heavy water, or rather the deuterium that is part of it, is an important participant in nuclear fusion reactions.The third isotope of hydrogen is tritium, designated by the symbol T. Unlike the first two, it is radioactive and is found in nature only in small quantities. In freshwater lakes the ratio between it and ordinary hydrogen is 1:10
18 , in surface waters 1:10 19 , it is absent in deep waters.see also HYDROGEN. ICE Ice, the solid phase of water, is used primarily as a refrigerant. It can be in equilibrium with the liquid and gaseous phases or only with the gaseous phase. A thick layer of ice has a bluish color, which is due to the way it refracts light. The compressibility of ice is very low.Ice at normal pressure exists only at a temperature of 0
° C or lower and has less density than cold water. This is why icebergs float in water. Moreover, since the ratio of the densities of ice and water at 0° Constantly, the ice always protrudes from the water by a certain part, namely 1/5 of its volume.see also ICEBERGS. STEAM Steam gaseous phase of water. Contrary to popular belief, he is invisible. That “steam” that escapes from a boiling kettle is actually many tiny droplets of water. Steam has properties that are very important for maintaining life on Earth. It is well known, for example, that under the influence of solar heat, water evaporates from the surface of seas and oceans. The resulting water vapor rises into the atmosphere and condenses, and then falls to the ground in the form of rain and snow. Without such a water cycle, our planet would have long ago turned into a desert.Steam has many uses. We are well acquainted with some, but have only heard about others. Among the most famous devices and mechanisms that use steam are irons, steam locomotives, steamships, and steam boilers. Steam rotates generator turbines in thermal power plants.
see also STEAM BOILER; THERMAL ENGINE; HEAT; THERMODYNAMICS.LITERATURE Eisenberg D., Kautsman V.Structure and properties of water . L., 1975Zatsepina G.N. Physical properties and structure of water . M., 1987
The three-dimensional state of liquid water is difficult to study, but much has been learned by analyzing the structure of ice crystals. Four neighboring hydrogen-bonded oxygen atoms occupy the vertices of a tetrahedron (tetra = four, hedron = plane). The average energy required to break such a bond in ice is estimated at 23 kJ/mol -1.
The ability of water molecules to form a given number of hydrogen chains, as well as the specified strength, creates an unusually high melting point. When it melts, it is held by liquid water, the structure of which is irregular. Most of the hydrogen bonds are distorted. To destroy the hydrogen-bonded crystal lattice of ice requires a large amount of energy in the form of heat.
Features of ice appearance (Ih)
Many ordinary people are wondering what kind of crystal lattice ice has. It should be noted that the density of most substances increases upon freezing, when molecular movements slow down and densely packed crystals form. The density of water also increases as it cools to its maximum at 4°C (277K). Then, when the temperature drops below this value, it expands.
This increase is due to the formation of an open hydrogen-bonded ice crystal with its lattice and lower density, in which each water molecule is tightly bound by the above element and four other values, and still moves fast enough to have more mass. As this action occurs, the liquid freezes from top to bottom. This has important biological consequences, whereby a layer of ice on a pond insulates living beings away from extreme cold. In addition, two additional properties of water are related to its hydrogen characteristics: specific heat capacity and evaporation.
Detailed description of structures
The first criterion is the amount required to raise the temperature of 1 gram of a substance by 1°C. Raising the degrees of water requires a relatively large portion of heat because each molecule is involved in numerous hydrogen bonds that must be broken for the kinetic energy to increase. By the way, the abundance of H 2 O in the cells and tissues of all large multicellular organisms means that temperature fluctuations inside the cells are minimized. This feature is critical because most biochemical reactions are rate sensitive.
Also significantly higher than many other liquids. To convert this solid into a gas requires a large amount of heat because the hydrogen bonds must be broken so that the water molecules can dislocate from each other and enter the said phase. Variable bodies are permanent dipoles and can interact with other similar compounds and those that are ionized and dissolved.
Other substances listed above can only come into contact if polarity is present. It is this compound that is involved in the structure of these elements. In addition, it can align around these particles formed from electrolytes, so that the negative oxygen atoms of the water molecules are oriented towards the cations, and the positive ions and hydrogen atoms are oriented towards the anions.
As a rule, molecular crystal lattices and atomic ones are formed. That is, if iodine is structured in such a way that I 2 is present in it, then in solid carbon dioxide, that is, in dry ice, there are CO 2 molecules at the nodes of the crystal lattice. When interacting with such substances, ice has an ionic crystal lattice. Graphite, for example, having an atomic structure based on carbon, is not able to change it, just like diamond.
What happens when a table salt crystal dissolves in water: polar molecules are attracted to the charged elements in the crystal, which leads to the formation of similar particles of sodium and chloride on its surface, as a result, these bodies dislocate from each other, and it begins to dissolve. From this we can observe that ice has a crystal lattice with ionic bonding. Each dissolved Na+ attracts the negative ends of several water molecules, while each dissolved Cl - attracts the positive ends. The shell surrounding each ion is called an escape sphere and usually contains several layers of solvent particles.

The variables or ion surrounded by elements are said to be sulfated. When water is the solvent, such particles become hydrated. Thus, any polar molecule tends to be solvation by elements of the liquid body. In dry ice, the type of crystal lattice forms atomic bonds in the aggregate state that are unchanged. Crystalline ice (frozen water) is another matter. Ionic organic compounds such as carboxylases and protonated amines must have solubility in hydroxyl and carbonyl groups. Particles contained in such structures move between molecules, and their polar systems form hydrogen bonds with this body.
Of course, the number of the latter groups in a molecule affects its solubility, which also depends on the reaction of the various structures in the element: for example, one-, two-, and three-carbon alcohols are miscible in water, but larger hydrocarbons with single hydroxyl compounds are much less dilute in liquids.
Hexagonal Ih is similar in shape to the atomic crystal lattice. For ice and all natural snow on Earth, it looks exactly like this. This is evidenced by the symmetry of the ice crystal lattice grown from water vapor (that is, snowflakes). Located in space group P 63/mm with 194; D 6h, Laue class 6/mm; similar to β-, which has a multiple of 6 helical axis (rotation around in addition to shear along it). It has a fairly open structure with low density, where the efficiency is low (~1/3) compared to simple cubic (~1/2) or face-centered cubic (~3/4) structures.
Compared to ordinary ice, the crystal lattice of dry ice, bound by CO 2 molecules, is static and changes only when atoms decay.

Description of lattices and their constituent elements
Crystals can be thought of as crystalline patterns consisting of sheets stacked on top of each other. Hydrogen bonding is ordered when in reality it is random, since protons can move between water (ice) molecules at temperatures above about 5 K. Indeed, it is likely that protons behave like a quantum fluid in a constant tunneling flow. This is enhanced by the scattering of neutrons showing their scattering density halfway between the oxygen atoms, indicating localization and coordinated motion. Here the similarity of ice with an atomic, molecular crystal lattice is observed.
The molecules have a stepped arrangement of the hydrogen chain in relation to their three neighbors in the plane. The fourth element has an eclipsed hydrogen bond arrangement. There is a slight deviation from perfect hexagonal symmetry, as much as 0.3% shorter in the direction of this chain. All molecules experience the same molecular environment. There is enough space inside each “box” to retain interstitial water particles. Although not generally considered, they have recently been effectively detected by neutron diffraction from powdered ice crystal lattice.
Change of substances
The hexagonal body has triple points with liquid and gaseous water 0.01 °C, 612 Pa, solid elements three -21.985 °C, 209.9 MPa, eleven and two -199.8 °C, 70 MPa, and -34 .7 °C, 212.9 MPa. The dielectric constant of hexagonal ice is 97.5.
The melting curve of this element is given by MPa. Equations of state are available, in addition to them some simple inequalities relating the change in physical properties with the temperature of hexagonal ice and its aqueous suspensions. Hardness varies with degrees, increasing from about or below gypsum (≤2) at 0°C, to feldspar levels (6 at -80°C, an abnormally large change in absolute hardness (>24 times).
The hexagonal crystal lattice of ice forms hexagonal plates and columns, where the top and bottom faces are the basal planes (0 0 0 1) with an enthalpy of 5.57 μJ cm -2, and the other equivalent side planes are called prism parts (1 0 -1 0) with 5.94 µJ cm -2. Secondary surfaces (1 1 -2 0) with 6.90 μJ ˣ cm -2 can be formed along the planes formed by the sides of the structures.
This structure shows an anomalous decrease in thermal conductivity with increasing pressure (like cubic and low-density amorphous ice), but differs from most crystals. This is due to a change in hydrogen bonding, which reduces the transverse speed of sound in the crystal lattice of ice and water.
There are methods that describe how to prepare large crystal samples and any desired ice surface. It is assumed that the hydrogen bond on the surface of the hexagonal body under study will be more ordered than inside the bulk system. Phase-lattice frequency-oscillating variational spectroscopy has shown that there is a structural asymmetry between the top two layers (L1 and L2) in the subsurface HO chain of the basal surface of hexagonal ice. The hydrogen bonds adopted in the upper layers of the hexagons (L1 O ··· HO L2) are stronger than those adopted in the second layer to the upper accumulation (L1 OH ··· O L2). Interactive hexagonal ice structures available.

Features of development
The minimum number of water molecules required for ice nucleation is approximately 275 ± 25, the same as for a complete icosahedral cluster of 280. Formation occurs at a factor of 10 10 at the air-water interface rather than in bulk water. The growth of ice crystals depends on different growth rates of different energies. Water must be protected from freezing during cryopreservation of biological samples, food and organs.
This is typically achieved by rapid cooling rates, the use of small samples and a cryoconservator, and increasing pressure to nucleate ice and prevent cell damage. The free energy of ice/liquid increases from ~30 mJ/m2 at atmospheric pressure to 40 mJ/m2 at 200 MPa, indicating the reason why this effect occurs.
Alternatively, they may grow more rapidly from prism surfaces (S2), on randomly disturbed surfaces of flash-frozen or disturbed lakes. The growth from the faces (1 1 -2 0) is at least the same, but turns them into the bases of a prism. Ice crystal development data have been fully explored. The relative growth rates of elements of different faces depend on the ability to form a greater degree of joint hydration. The (low) temperature of the surrounding water determines the degree of branching in the ice crystal. Particle growth is limited by the rate of diffusion at low degrees of supercooling, i.e.<2 ° C, что приводит к большему их количеству.

But it is limited by development kinetics at higher levels of lowering degrees >4°C, which leads to needle-like growth. This form is similar to the structure of dry ice (has a crystal lattice with a hexagonal structure), different characteristics of surface development and the temperature of the surrounding (supercooled) water that lies behind the flat forms of snowflakes.
The formation of ice in the atmosphere profoundly influences the formation and properties of clouds. Feldspars, found in desert dust that enters the atmosphere by the millions of tons per year, are important formatives. Computer simulations have shown that this is due to the nucleation of planes of prismatic ice crystals on high-energy surface planes.
Some other elements and lattices
Solutes (except for a very small amount of helium and hydrogen, which may enter interstices) cannot be incorporated into the Ih structure at atmospheric pressure, but are forced to the surface or an amorphous layer between the particles of the microcrystalline body. At the sites of the crystal lattice of dry ice there are some other elements: chaotropic ions, such as NH 4 + and Cl -, which are included in the freezing of the liquid more easily than other kosmotropic ones, such as Na + and SO 4 2-, so their removal is impossible, due to the fact that they form a thin film of the remaining liquid between the crystals. This can lead to electrical charging of the surface due to the dissociation of surface water balancing the remaining charges (which can also result in magnetic radiation) and a change in the pH of the residual liquid films, for example NH 4 2 SO 4 becoming more acidic and NaCl becoming more alkaline.
They are perpendicular to the faces of the ice crystal lattice, showing the attached next layer (with O-black atoms). They are characterized by a slowly growing basal surface (0 0 0 1), where only isolated water molecules are attached. A rapidly growing (1 0 -1 0) surface of a prism, where pairs of newly attached particles can bond with each other with hydrogen (one bond/two molecules of the element). The fastest growing face is (1 1 -2 0) (secondary prismatic), where chains of newly attached particles can interact with each other by hydrogen bonding. One of its chain/element molecule is a form that forms ridges that divide and encourage the transformation into two sides of the prism.

Zero point entropy
kBˣ Ln ( N
Scientists and their works in this field
Can be defined as S 0 = kBˣ Ln ( N E0), where k B is Boltzmann's constant, N E is the number of configurations at energy E, and E0 is the lowest energy. This value for the entropy of hexagonal ice at zero kelvin does not violate the third law of thermodynamics, “The entropy of an ideal crystal at absolute zero is exactly zero,” since these elements and particles are not ideal and have disordered hydrogen bonding.
In this body, hydrogen bonding is random and rapidly changing. These structures are not exactly equal in energy, but extend to a very large number of energetically close states and obey the “rules of ice.” Zero point entropy is the disorder that would remain even if the material could be cooled to absolute zero (0 K = -273.15 °C). Gives rise to experimental confusion for hexagonal ice 3.41 (±0.2) ˣ mol -1 ˣ K -1 . Theoretically, it would be possible to calculate the zero entropy of known ice crystals with much greater accuracy (neglecting defects and energy level scatter) than determining it experimentally.

Although the order of protons in bulk ice is not ordered, the surface probably prefers the order of said particles in the form of bands of dangling H atoms and O lone pairs (zero entropy with ordered hydrogen bonds). The disorder of the zero point ZPE, J ˣ mol -1 ˣ K -1 and others was found. From all of the above, it is clear and understandable what types of crystal lattices are characteristic of ice.
Option #1.
1. Are ice and water molecules different from each other?
1) they are the same; 2) the ice molecule is colder; 3) the ice molecule is smaller;
4) the water molecule is smaller
2. What is diffusion?
Molecules of another; 3) chaotic movement of molecules of matter;
4) mixing substances
4. When a substance cools, the molecules move:
Kind of substance
5. The speed of movement of hydrogen molecules has increased. Wherein
Temperature …
No answer
6. If you pour water from a glass into a plate, then...
Shape and volume
7. In which water does diffusion occur faster?
Happening
8. In which substances does diffusion occur more slowly when od-
Under what conditions?
All substances
9. Molecules of a substance are located at large distances,
Are strongly attracted and oscillate around the equilibrium position
This substance...
1) gaseous; 2) liquid; 3) hard; 4) such a substance does not exist
Option number 2.
1. Are the molecules of ice and water vapor different from each other?
1) the ice molecule is colder; 2) they are the same; 3) ice molecule
Less; 4) the ice molecule is larger
2. Diffusion is...
1) penetration of molecules of one substance into molecules of another;
2) penetration of molecules of one substance into the spaces between
Molecules of another; 3) chaotic movement of molecules of substances
Va; 4) mixing substances
3. Between the molecules of any substance there is:
1) mutual attraction; 2) mutual repulsion; 3) mutual
Attraction and repulsion; 4) different substances have different
4. When water is heated, molecules move:
1) at the same speed; 2) slower; 3) faster; 4) depends on
Kind of substance
5. The speed of movement of oxygen molecules has decreased. Wherein
Temperature …
1) has not changed; 2) decreased; 3) increased; 4) correct
No answer
6. If you pour water from a plate into a glass, then...
1) the shape and volume of water will change; 2) the shape will change, the volume will change
Stored; 3) the shape will remain the same, the volume will change; 4) will be preserved
Volume and shape
7. In which water does diffusion occur more slowly?
1) in cold; 2) hot; 3) the same; 4) diffusion in water is not
Happening
8. In which substances does diffusion occur faster at the same
What are your conditions?
1) in gaseous; 2) in liquid; 3) in solids; 4) the same in
All substances
9. Molecules of a substance are located at short distances, strongly
They attract and oscillate around the equilibrium position. This
Substance...
1) gaseous; 2) liquid; 3) hard; 4) there is no such substance
Exists
V.V. Makhrova, GS(K)OU S(K)OSH (VII type) N 561, St. Petersburg