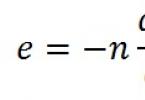Lipids resynthesized in the intestine are transported as part of chylomicrons with lymph. Lipids are insoluble in water, so they are transported in association with proteins.
Lipoproteins are complexes of proteins and lipids, the transport form of lipids in the blood. LP lipids: triglycerides, phospholipids, cholesterol. LP proteins are apoproteins of individual classes of LP.
The functions of apoproteins are structural (LP), transport, secretory (needed for the secretion of LP by liver and intestinal cells), necessary for the interaction of LP with receptors, activate enzymes involved in the metabolism of LP, impart water solubility to lipids, apoprotein A1 in HDL activates LCAT.
Lipoprotein structure. The hydrophobic core (cholesterol esters, TG) is surrounded on the outside by phospholipids, free cholesterol, and apoproteins.
Classification of drugs.
based on mobility in the electric field: - ChMs remain at the start, - others migrate to the globulin zones: ß-LP, pre-ß-LP, α-LP.
According to the hydrated density (using the ultracentrifugation method), drugs are divided into CM, VLDL, LDPP, LDL, and HDL.
Biological role of drugs. Endogenous TG are delivered to peripheral cells to meet energy needs, and endogenous cholesterol is delivered to membrane biosynthesis.
Composition and properties of lipoproteins.
Chylomicrons transport exogenous TG, cholesterol, phospholipids and dietary fats from the intestine to tissues through the lymphatic system. Immature CMs are synthesized in enterocytes, which first enter the lymph and then the bloodstream. The main apoprotein of CM, protein B-48, is synthesized in the cells of the intestinal mucosa and is necessary for the formation of the structure of CM. In the blood, immature CM receive other apoproteins from HDL - C- and E- and are converted into mature CM. The first organ through which chemotherapy must pass is the lungs. When CM enters the bloodstream from the intestine, mast cells are activated with the release of heparin and activation of lipoprotein lipase.
Adsorptive lipemia is an increase in the amount of lipids in the blood that occurs after eating.
Lipoprotein lipase (clearing factor) hydrolyzes TAG into CM and VLDL, is located in the endothelium of the capillaries of various organs, is activated by heparin and an increase in TAG in the blood. TAG chylomicrons are cleaved on the surface and inside hepatocytes, on the surface of the endothelium of adipose tissue capillaries.
VLDL and HDL are secreted into the blood by the liver, where they are synthesized. LDL is formed in the bloodstream from VLDL as a result of hydrolysis of the TG portion of VLDL by lipoprotein lipase.
The fate of LDL. There are receptors for LDL on the plasma membranes of cells. LDL penetrates into cells, where, under the influence of lysosomal hydrolases, they break down into their constituent components, free cholesterol is included in the plasma membrane or is esterified and deposited in the form of esters in the cytoplasm. Nonspecific endocytosis of LDL is possible.
HDL carries cholesterol to the liver. In the liver, cholesterol is oxidized into bile acids and removed through the intestines. Cholesterol oxidation occurs in the liver by the monooxygenase system. Cholesterol 7a-hydroxylase is a rate-limiting enzyme. HDL is capable of accepting cholesterol from cell membranes.
Conversion of free cholesterol into esterified: cholesterol + lecithin → lysolecithin + cholesterol ester. Cholesterol ester is formed on the surface of HDL and transferred to the HDL core.
A decrease in HDL cholesterol in the blood plasma is associated with a decrease in LCAT, the number of HDL particles, lecithin, and apoprotein A1.
Half life. HM – less than an hour, VLDL – 2-4 hours, LDL – 2-4 days, HDL – 5 days. LDL and HDL are absorbed by endocytosis by the cells of the liver, intestines, adipose tissue, kidneys, and adrenal glands and are destroyed in lysosomes.
Non-esterified fatty acids (NEFA). Fatty acids in blood plasma are in esterified form: in the composition of phospholipids, cholesterol esters, mono-, di-, triglycerides. In free form, fatty acids are transported in plasma from adipose tissue and liver to skeletal muscle, in which case they are bound to albumin.
NEFA enter the blood plasma as a result of lipolysis of TG, catalyzed by lipase in adipose tissue; they are formed by the action of lipoprotein lipase on TG of the blood plasma during their transition into tissues; fatty acids with a chain length of less than 1o carbon atoms are absorbed in non-esterified form through the portal circulatory system and enter to the liver (this is important for children, since milk is rich in short-chain fatty acids).
Triacylglycerides are a transport form for saturated fatty acids. Phospholipids and cholesterol are a transport form for polyunsaturated fatty acids.
Functions of NEFA - provide 50% of energy during fasting, energy material for the myocardium, muscles, kidneys, liver, saturated fatty acids perform energy, and unsaturated fatty acids perform plastic functions.
Answer. Ammonia, glutamine, and ammonium ion NH 4 + increase in the patient’s blood.
Lipid transport in the body occurs in two ways:
- 1) fatty acids are transported in the blood with the help of albumins;
- 2) TG, FL, HS, EHS, etc. Lipids are transported in the blood as part of lipoproteins.
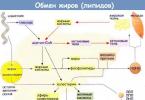
Lipoprotein metabolism
Lipoproteins (LP) are spherical supramolecular complexes consisting of lipids, proteins and carbohydrates. LPs have a hydrophilic shell and a hydrophobic core. The hydrophilic shell includes proteins and amphiphilic lipids - PL, cholesterol. The hydrophobic core includes hydrophobic lipids - TG, cholesterol esters, etc. LPs are highly soluble in water.
Several types of lipids are synthesized in the body; they differ in chemical composition, are formed in different places and transport lipids in different directions.
Medicines are separated using:
- 1) electrophoresis, by charge and size, on b-LP, v-LP, pre-c-LP and CM;
- 2) centrifugation, by density, for HDL, LDL, LDLP, VLDL and CM.
The ratio and amount of LP in the blood depends on the time of day and nutrition. During the post-absorptive period and during fasting, only LDL and HDL are present in the blood.
Main types of lipoproteins
Composition, % VLDL CM
- (pre-in-LP) DILI
- (pre-in-LP) LDL
- (v-LP) HDL
- (b-LP)
Proteins 2 10 11 22 50
FL 3 18 23 21 27
EHS 3 10 30 42 16
TG 85 55 26 7 3
Density, g/ml 0.92-0.98 0.96-1.00 0.96-1.00 1.00-1.06 1.06-1.21
Diameter, nm >120 30-100 30-100 21-100 7-15
Functions Transport of exogenous food lipids to tissues Transport of endogenous liver lipids to tissues Transport of endogenous liver lipids to tissues Transport of cholesterol
in tissue Removal of excess cholesterol
from fabrics
apo A, C, E
Place of formation enterocyte hepatocyte in the blood from VLDL in the blood from LDLP hepatocyte
Apo B-48, C-II, E B-100, C-II, E B-100, E B-100 A-I C-II, E, D
Normal in blood< 2,2 ммоль/л 0,9- 1,9 ммоль/л
Apobelki
The proteins that make up the drug are called apoproteins (apoproteins, apo). The most common apoproteins include: apo A-I, A-II, B-48, B-100, C-I, C-II, C-III, D, E. Apoproteins can be peripheral (hydrophilic: A-II, C-II, E) and integral (have a hydrophobic region: B-48, B-100). Peripheral apos transfer between LPs, but integral apos do not. Apoproteins perform several functions:
Apoprotein Function Place of formation Localization
A-I Activator LCAT, formation of ECS liver HDL
A-II Activator of LCAT, formation of ECS HDL, CM
B-48 Structural (LP synthesis), receptor (LP phagocytosis) enterocyte XM
B-100 Structural (LP synthesis), receptor (LP phagocytosis) liver VLDL, LDPP, LDL
C-I Activator LCAT, formation of ECS Liver HDL, VLDL
C-II LPL activator, stimulates the hydrolysis of TG in the lipoprotein Liver HDL > CM, VLDL
C-III LPL inhibitor, inhibits the hydrolysis of TG in the LP Liver HDL > CM, VLDL
D Cholesteryl ester transfer (CET) Liver HDL
E Receptor, phagocytosis LP liver HDL > CM, VLDL, LDLP
Lipid transport enzymes
Lipoprotein lipase (LPL) (EC 3.1.1.34, LPL gene, about 40 defective alleles) is associated with heparan sulfate located on the surface of endothelial cells of blood vessel capillaries. It hydrolyzes TG in the composition of the drug to glycerol and 3 fatty acids. With the loss of TG, CM turn into residual CM, and VLDL increases its density to LDLP and LDL.
Apo C-II LP activates LPL, and LP phospholipids are involved in the binding of LPL to the surface of the LP. LPL synthesis is induced by insulin. Apo C-III inhibits LPL.
LPL is synthesized in the cells of many tissues: fat, muscle, lungs, spleen, cells of the lactating mammary gland. It is not in the liver. LPL isoenzymes of different tissues differ in Km values. In adipose tissue, LPL has Km 10 times greater than in the myocardium, therefore, adipose tissue absorbs fatty acids only when there is an excess of TG in the blood, and the myocardium constantly, even with a low concentration of TG in the blood. Fatty acids in adipocytes are used for the synthesis of TG, in the myocardium as a source of energy.
Hepatic lipase is located on the surface of hepatocytes; it does not act on mature cholesterol, but hydrolyzes TG in the LDPP.
Lecithin: cholesterol acyl transferase (LCAT) is located in HDL, it transfers acyl from lecithin to cholesterol to form ECL and lysolecithin. It is activated by apo A-I, A-II and C-I.
lecithin + CS > lysolecithin + ECS
ECS is immersed in the HDL core or transferred with the participation of apo D to other HDL.
Lipid transport receptors
The LDL receptor is a complex protein consisting of 5 domains and containing a carbohydrate part. The LDL receptor interacts with the proteins ano B-100 and apo E, binds LDL well, worse than DILI, VLDL, and residual CM containing these apos. Tissue cells contain a large number of LDL receptors on their surface. For example, one fibroblast cell has from 20,000 to 50,000 receptors.
If the amount of cholesterol entering a cell exceeds its need, then the synthesis of LDL receptors is suppressed, which reduces the flow of cholesterol from the blood into the cells. When the concentration of free cholesterol in the cell decreases, on the contrary, the synthesis of HMG-CoA reductase and LDL receptors is activated. Hormones stimulate the synthesis of LDL receptors: insulin and triiodothyronine (T3), sex hormones, and glucocorticoids reduce it.
LDL receptor-like protein On the surface of cells in many organs (liver, brain, placenta), there is another type of receptor called “LDL receptor-like protein.” This receptor interacts with apo E and captures remnant (residual) CM and DILI. Since remnant particles contain cholesterol, this type of receptor also ensures its entry into tissues.
In addition to the entry of cholesterol into tissues by endocytosis of lipoproteins, a certain amount of cholesterol enters cells by diffusion from LDL and other lipoproteins upon their contact with cell membranes.
The normal concentration in the blood is:
- * LDL
- * total lipids 4-8g/l,
- * TG 0.5-2.1 mmol/l,
- * Free fatty acids 400-800 µmol/l
Lipids are insoluble in an aqueous environment, therefore, for their transport in the body, complexes of lipids with proteins are formed - lipoproteins (LP). There are exo- and endogenous lipid transport. Exogenous includes the transport of lipids received from food, and endogenous includes the movement of lipids synthesized in the body.
There are several types of LP, but they all have a similar structure - a hydrophobic core and a hydrophilic layer on the surface. The hydrophilic layer is formed by proteins called apoproteins and amphiphilic lipid molecules - phospholipids and cholesterol. The hydrophilic groups of these molecules face the aqueous phase, and the hydrophobic groups face the core, in which the transported lipids are located. Apoproteins perform several functions:
· form the structure of lipoproteins (for example, B-48 is the main protein of XM, B-100 is the main protein of VLDL, LDPP, LDL);
· interact with receptors on the surface of cells, determining which tissues will capture this type of lipoprotein (apoprotein B-100, E);
· are enzymes or activators of enzymes acting on lipoproteins (C-II - activator of lipoprotein lipase, A-I - activator of lecithin: cholesterol acyltransferase).
During exogenous transport, TAGs resynthesized in enterocytes together with phospholipids, cholesterol and proteins form CM, and in this form are secreted first into the lymph and then into the blood. In lymph and blood, apoproteins E (apo E) and C-II (apo C-II) are transferred from HDL to CM, thus turning CM into “mature” ones. ChMs are quite large in size, so after eating a fatty meal they give the blood plasma an opalescent, milk-like appearance. Once in the circulatory system, CMs quickly undergo catabolism and disappear within a few hours. The time of destruction of CM depends on the hydrolysis of TAG under the action of lipoprotein lipase (LPL). This enzyme is synthesized and secreted by adipose and muscle tissues, and mammary gland cells. Secreted LPL binds to the surface of the endothelial cells of the capillaries of the tissues where it was synthesized. The regulation of secretion is tissue specific. In adipose tissue, LPL synthesis is stimulated by insulin. This ensures the supply of fatty acids for synthesis and storage in the form of TAG. In diabetes mellitus, when there is a deficiency of insulin, LPL levels decrease. As a result, a large amount of LP accumulates in the blood. In muscles, where LPL is involved in supplying fatty acids for oxidation between meals, insulin inhibits the formation of this enzyme.
On the surface of CM, there are 2 factors necessary for LPL activity: apoC-II and phospholipids. ApoC-II activates this enzyme, and phospholipids are involved in binding the enzyme to the surface of CM. As a result of the action of LPL on TAG molecules, fatty acids and glycerol are formed. The bulk of fatty acids penetrate into tissues, where they can be deposited in the form of TAG (adipose tissue) or used as an energy source (muscles). Glycerol is transported by the blood to the liver, where during the absorption period it can be used for the synthesis of fats.
As a result of the action of LPL, the amount of neutral fats in CM decreases by 90%, particle sizes decrease, and apoC-II is transferred back to HDL. The resulting particles are called residual CM (remnants). They contain PL, cholesterol, fat-soluble vitamins, apoB-48 and apoE. Residual CMs are captured by hepatocytes, which have receptors that interact with these apoproteins. Under the action of lysosome enzymes, proteins and lipids are hydrolyzed and then utilized. Fat-soluble vitamins and exogenous cholesterol are used in the liver or transported to other organs.
During endogenous transport, TAG and PL resynthesized in the liver are included in VLDL, which includes apoB100 and apoC. VLDL is the main transport form for endogenous TAG. Once in the blood, VLDL receives apoC-II and apoE from HDL and is exposed to LPL. During this process, VLDL is first converted into LDLP and then into LDL. The main lipid of LDL becomes cholesterol, which in their composition is transferred to the cells of all tissues. The fatty acids formed during hydrolysis enter the tissues, and glycerol is transported by the blood to the liver, where it can again be used for the synthesis of TAG.
All changes in the content of drugs in the blood plasma, characterized by their increase, decrease or complete absence, are combined under the name dislipoproteinemia. Dyslipoproteinemia can be either a specific primary manifestation of disorders in the metabolism of lipids and lipoproteins, or a concomitant syndrome in certain diseases of internal organs (secondary dyslipoproteinemia). With successful treatment of the underlying disease, they disappear.
Hypolipoproteinemia includes the following conditions.
1. Abetalipoproteinemia occurs due to a rare hereditary disease - a defect in the apoprotein B gene, when the synthesis of proteins apoB-100 in the liver and apoB-48 in the intestine is disrupted. As a result, CMs are not formed in the cells of the intestinal mucosa, and VLDL is not formed in the liver, and droplets of fat accumulate in the cells of these organs.
2. Familial hypobetalipoproteinemia: the concentration of drugs containing apoB is only 10-15% of the normal level, but the body is capable of forming cholesterol.
3. Familial a-LP deficiency (Tangier disease): practically no HDL is found in the blood plasma, and a large amount of cholesterol esters accumulates in the tissues; patients lack apoC-II, which is an activator of LPL, which leads to an increase in TAG concentration characteristic of this condition in blood plasma.
Among hyperlipoproteinemias, the following types are distinguished.
Type I - hyperchylomicronemia. The rate of removal of CM from the bloodstream depends on the activity of LPL, the presence of HDL, which supplies apoproteins C-II and E for CM, and the activity of transferring apoC-II and apoE to CM. Genetic defects of any of the proteins involved in the metabolism of CMs lead to the development of familial hyperchylomicronemia - the accumulation of CMs in the blood. The disease manifests itself in early childhood and is characterized by hepatosplenomegaly, pancreatitis, and abdominal pain. As a secondary symptom, it is observed in patients with diabetes mellitus, nephrotic syndrome, hypothyroidism, and also with alcohol abuse. Treatment: diet low in lipids (up to 30 g/day) and high in carbohydrates.
Type II – familial hypercholesterolemia (hyper-b-lipoproteinemia). This type is divided into 2 subtypes: IIa, characterized by a high level of LDL in the blood, and IIb, with increased levels of both LDL and VLDL. The disease is associated with impaired reception and catabolism of LDL (defect in cellular receptors for LDL or changes in the structure of LDL), accompanied by increased biosynthesis of cholesterol, apo-B and LDL. This is the most serious pathology in drug metabolism: the risk of developing coronary artery disease in patients with this type of disorder increases 10-20 times compared to healthy individuals. As a secondary phenomenon, type II hyperlipoproteinemia can develop with hypothyroidism and nephrotic syndrome. Treatment: Diet low in cholesterol and saturated fat.
Type III - dys-b-lipoproteinemia (broadband betalipoproteinemia) is caused by an abnormal composition of VLDL. They are enriched with free cholesterol and defective apo-E, which inhibits the activity of hepatic TAG lipase. This leads to disturbances in the catabolism of cholesterol and VLDL. The disease manifests itself at the age of 30-50 years. The condition is characterized by a high content of VLDL residues, hypercholesterolemia and triacylglycerolemia, xanthomas, atherosclerotic lesions of peripheral and coronary vessels are observed. Treatment: diet therapy aimed at weight loss.
Type IV – hyperpre-b-lipoproteinemia (hypertriacylglycerolemia). The primary variant is due to a decrease in LPL activity; an increase in the level of TAG in the blood plasma occurs due to the VLDL fraction; accumulation of CM is not observed. It occurs only in adults and is characterized by the development of atherosclerosis, first of the coronary, then of the peripheral arteries. The disease is often accompanied by decreased glucose tolerance. As a secondary manifestation, it occurs in pancreatitis and alcoholism. Treatment: diet therapy aimed at weight loss.
Type V – hyperpre-b-lipoproteinemia with hyperchylomicronemia. With this type of pathology, changes in blood lipid fractions are complex: the content of cholesterol and VLDL is increased, the severity of LDL and HDL fractions is reduced. Patients are often overweight; hepatosplenomegaly and pancreatitis may develop; atherosclerosis does not develop in all cases. As a secondary phenomenon, type V hyperlipoproteinemia can be observed in insulin-dependent diabetes mellitus, hypothyroidism, pancreatitis, alcoholism, and type I glycogenosis. Treatment: diet therapy aimed at weight loss, a diet low in carbohydrates and fats.
After absorption into the intestinal epithelium free fatty acids and 2-monoglycerides again form triglycerides and, together with phospholipids and cholesterol, are included in chylomicrons. Chylomicrons are transported with the lymph flow through the thoracic duct into the superior vena cava, thus entering the general bloodstream.
Inside a chylomicron triglycerides hydrolyzed by lipoprotein lipase, which leads to the release of fatty acids on the surface of blood capillaries in tissues. This causes the transport of fatty acids into tissues and the subsequent formation of chylomicron residues depleted in triglycerides. These residues then take up cholesterol esters from high-density lipoproteins, and the particles are quickly taken up by the liver. This transport system for food-derived fatty acids is called the exogenous transport system.
There is also endogenous transport system, intended for intraorgan transport of fatty acids formed in the body itself. Lipids are transported from the liver to peripheral tissues and back, and are also transferred from fat depots to various organs. The transport of lipids from the liver to peripheral tissues involves the concerted actions of VLDL, intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). VLDL particles, like chylomicrons, consist of a large hydrophobic core formed by triglycerides and cholesterol esters and a surface lipid layer consisting mainly of phospholipids and cholesterol.
VLDL are synthesized in the liver, and fat deposition in peripheral tissues is their main function. Once VLDL enters the bloodstream, it is exposed to lipoprotein lipase, which hydrolyzes triglycerides to free fatty acids. Free fatty acids derived from chylomicrons or VLDL can be used as energy sources, structural components of phospholipid membranes, or converted back to triglycerides and stored as such. Chylomicron triglycerides and VLDL are also hydrolyzed by liver lipase.
Particles VLDL through hydrolysis of triglycerides are converted into denser, smaller cholesterol- and triglyceride-rich residues (LCRs), which are removed from the plasma by hepatic lipoprotein receptors or can be converted to LDL. LDL is the main lipoprotein carrier of cholesterol.
The return from peripheral tissues to the liver is often called reverse cholesterol transport. HDL particles participate in this process by taking cholesterol from tissues and other lipoproteins and transporting it to the liver for subsequent excretion. Another type of transport that exists between organs is the transfer of fatty acids from fat depots to organs for oxidation.
Fatty acid, obtained mainly as a result of the hydrolysis of triglycerides in adipose tissue, are secreted into the plasma, where they combine with albumin. Albumin-bound fatty acids are transported along a concentration gradient to tissues with active metabolism, where they are used primarily as energy sources.
Over the past 20 years, only a few research were devoted to the issue of lipid transport in the perinatal period (the results of these studies are not presented in this publication). The need for a more detailed study of this problem is obvious.
Fatty acids are used as building blocks material as part of cell wall lipids, as energy sources, and are also stored “in reserve” in the form of triglycerides, mainly in adipose tissue. Some omega-6 and omega-3 LCPUFAs are precursors to bioactive metabolites used in cell signaling, gene regulation, and other metabolically active systems.
Question about the role LCPUFA ARA and DHA in the process of child growth and development has been one of the most important issues in research conducted in the field of pediatric nutrition over the past two decades.
Lipids are one of the main components of cell membranes. A significant amount of research in the field of lipid physiology has focused on two fatty acids - ARA and DHA. ARA is found in the cell membranes of all structures of the human body; it is a precursor of series 2 eicosanoids, series 3 leukotrienes and other metabolites that are involved in cell signaling systems and the process of gene regulation. Research on DHA often points to its structural and functional roles in cell membranes.
This fatty acid found in high concentrations in the gray matter of the brain, as well as in the rods and cones of the retina. Studies on phasing out omega-3 fatty acids in animals have shown that 22-carbon omega-6 LCPUFAs (e.g., 22:5 n-6) can structurally, but not functionally, replace 22:6 n-3. With inadequate levels of 22:6 n-3 in tissues, visual and cognitive impairments are detected. Altering tissue 22:6 n-3 levels has been shown to affect neurotransmitter function, ion channel activity, signaling pathways, and gene expression.

Return to section table of contents "
Since lipids are basically hydrophobic molecules, they are transported in the aqueous phase of the blood as part of special particles - lipoproteins.
The structure of transport lipoproteins can be compared with nut who have shell And core. The “shell” of the lipoprotein is hydrophilic, the core is hydrophobic.
- the surface hydrophilic layer is formed phospholipids(their polar part), cholesterol(his OH group), squirrels. The hydrophilicity of the surface layer lipids is designed to ensure the solubility of the lipoprotein particle in the blood plasma,
- the "core" is formed by non-polar cholesterol esters(HS) and triacylglycerols(TAG), which are transported fats. Their ratio varies in different types of lipoproteins. Also facing the center are the fatty acid residues of phospholipids and the cyclic part of cholesterol.
Scheme of the structure of any transport lipoprotein
There are four main classes of lipoproteins:
- high density lipoproteins (HDL, α-lipoproteins, α-LP),
- low-density lipoproteins (LDL, β-lipoproteins, β-LP),
- very low density lipoproteins (VLDL, pre-β-lipoproteins, pre-β-LP),
- chylomicrons (CM).
The properties and functions of lipoproteins of different classes depend on their composition, i.e. on the type of proteins present and on the ratio of triacylglycerols, cholesterol and its esters, phospholipids.

Comparison of the size and properties of lipoproteins
Functions of lipoproteins
The functions of blood lipoproteins are
1. Transfer to cells of tissues and organs
- saturated and monounsaturated fatty acids in the composition of triacylglycerols for subsequent storage or use as energy substrates,
- polyunsaturated fatty acids in cholesterol esters for use by cells in the synthesis of phospholipids or the formation of eicosanoids,
- cholesterol as a membrane material,
- phospholipids as membrane material,
Chylomicrons and VLDL are primarily responsible for transport fatty acids as part of TAG. High and low density lipoproteins - for the transport of free cholesterol And fatty acids as part of its ethers. HDL is also capable of donating part of its phospholipid membrane to cells.
2. Removal of excess cholesterol from cell membranes.
3. Transport of fat-soluble vitamins.
4. Transfer of steroid hormones (along with specific transport proteins).
Lipoprotein apoproteins
The proteins in lipoproteins are usually called apowhites, there are several types of them - A, B, C, D, E. In each class of lipoproteins there are corresponding apoproteins that perform their own function:
1. Structural function(" stationary» proteins) – bind lipids and form protein-lipid complexes:
- apoB-48– adds triacylcerols,
- apoB-100– binds both triacylglycerols and cholesterol esters,
- apoA-I– accepts phospholipids,
- apoA-IV– binds to cholesterol.
2. Cofactor function(" dynamic» proteins) – affect the activity of lipoprotein metabolic enzymes in the blood.