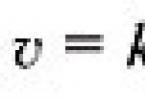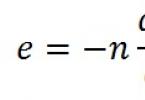Chemical reaction rate
and its dependence on various factors
Lesson using information technology
It is impossible to learn chemistry in any case,
without seeing the practice itself and without taking on chemical operations.
M.V. Lomonosov
The restructuring of higher and secondary specialized education in the country and school reform provide for further improvement of forms, methods and means of teaching, the use of a variety of technologies, including person-centered learning (PLL), problem-search and computer technologies.
We, teachers, are also changing. In my work I try to constantly use new developments and modern educational technologies.
Recently, a lot of materials have appeared on computer disks. They can be used in developing essays, writing term papers, and in students’ independent work. Information technologies allow me to quickly organize training and testing of knowledge, create adaptive programs and apply them in teaching chemistry.
Computer technology and the use of computer technologies today act not only as a means of automating all learning processes, but also as a tool for dramatically increasing the efficiency of students' intellectual activity.
I use computer technology in my lessons for different purposes:
Problem solving, quantitative calculations, data processing (according to the proposed algorithm);
Carrying out self-control and standardized control of knowledge on the content of educational information (tests, control differentiated tasks, maps and other questionnaires);
Automation of a chemical experiment, connection with optical equipment (projection of experiments onto a screen);
Obtaining the necessary reference data, preparing tests, differentiated works, analyzing typical mistakes of students (automated control systems and information banks);
Independent work of students to develop abstracts and term papers, work with the material, perform test work (once receiving the result, exercise self-control).
The proposed lesson from the section “Chemical Kinetics” corresponds to the program of the textbook “Chemistry-10” by the authors L.S. Guzey and R.P. Surovtseva. The study of this topic is preceded by the study of the thermodynamics of reactions. The proposed material does not correspond to the required minimum content, but primarily to the profile level of training.
The lesson uses group work, a differentiated approach, developmental and problem-search technologies, and most importantly, computer technology to conduct a demonstration experiment, which allows you to clearly understand what the rate of a chemical reaction is and how it depends on various factors.
Lesson objectives. Update and deepen knowledge about the rate of chemical reactions; using group work, consider and study various factors: the nature of the reacting substances, the surface area of contact of the substances, temperature, catalyst; using a computer measuring unit, clearly demonstrate what the rate of a chemical reaction is and how it depends on the concentration of the reacting substances.
Lesson motto.“There is only what can be measured” (M. Planck).
Class design. The teacher informs in advance the topic of the upcoming lesson, divides the class into four creative groups of 5-6 people, approximately equal in abilities. In the previous lesson, students receive homework - to prepare reports on the practical application of the Arrhenius equation and the types of catalysis.
Equipment and reagents. On the students' desks - textbooks, notebooks, tables, laboratory sheets, racks with test tubes;
group 1: zinc granules, magnesium tape, hydrochloric acid solution;
group 2: glass rod; iron filings, iron nail, copper(II) chloride solution;
group 3: pipette, test tube holder, alcohol lamp, matches; copper(II) oxide, sulfuric acid solution;
group 4(performs a demonstration experiment on a demonstration table): a computer with a measuring unit, an optical density sensor at a wavelength of 525 nm, a cuvette, a magnetic stirrer, a 10 ml syringe, a 100 ml graduated cylinder; solutions of potassium iodide KI 1M, potassium persulfate K 2 S 2 O 8 0.1 M, distilled water.
Students make all notes during the lesson in their notebooks.
DURING THE CLASSES
Motivation for the importance of the chosen topic
The teacher begins explaining the material with examples of chemical reactions occurring at different rates. Students can give examples of reactions.
Chemical reactions occur at different rates. Some progress slowly, over months, such as the corrosion of iron or the fermentation of grape juice, which results in wine. Others are completed in a few weeks, like the alcoholic fermentation of glucose. Still others end very quickly, such as the precipitation of insoluble salts, and some occur instantly, such as explosions.
Almost instantly, very quickly, many reactions occur in aqueous solutions:
Let's mix aqueous solutions of Na 2 CO 3 and CaCl 2, the reaction product CaCO 3 is insoluble in water and is formed immediately;
If we add excess acid to an alkaline solution of phenolphthalein, the solution becomes discolored instantly. This means that the neutralization reaction, the reaction of converting the colored form of the indicator into a colorless one, proceeds very quickly.
Rust forms slowly on iron objects. Black-brown or greenish-colored corrosion products (patina) slowly form on copper and bronze objects. The speed of all these processes is different.
Updating Views
about the speed of chemical reactions
Chemical reactions are one of the most important concepts in chemistry. To understand them and competently use them in the educational process, the teacher needs to know and be able to explain the main characteristics of any chemical reaction: thermal effect, equilibrium, speed. Chemical thermodynamics makes it possible to predict in which direction a particular chemical reaction may spontaneously proceed, but chemical thermodynamics alone does not answer the question of how and at what speed the reaction will proceed. The concept of the rate of a chemical reaction is one of the basic ones in chemical kinetics.
To study new material, students use the necessary knowledge about the rate of a chemical reaction; they go through the stage of updating their knowledge. But this concept is deepened by the concepts of the speed of homogeneous and heterogeneous reactions, activation energy, the Arrhenius equation is introduced - this is the zone of proximal development of students (see Appendix No. 1 “Structure of problem-search activity of teachers and students...”).
What do you mean by reaction speed? How can it be measured and changed? The science that studies the patterns of reactions over time - chemical kinetics - will help answer these questions.
Let us recall the basic concepts and patterns used in kinetics (students answer and teacher supplements).
Chemical kinetics is a branch of chemistry whose task is to explain qualitative and quantitative changes in chemical processes that occur over time. Usually this general task is divided into two, more specific ones:
1) identifying the reaction mechanism - establishing the elementary stages of the process and the sequence of their occurrence (qualitative changes);
2) quantitative description of a chemical reaction - the establishment of strict relationships that make it possible to calculate changes in the amounts of initial reagents and products as the reaction proceeds.
The main concept in chemical kinetics is the concept of reaction rate. Chemical reaction rate is determined by the amount of substance reacted per unit time in a unit of reaction space.
If the concentration of one of the reactants decreases from With 1 to With 2 for the period from t 1 to t 2, then in accordance with the definition of the reaction rate is equal to (Fig. 1):
The “–” sign on the right side of the equation means the following. As the reaction progresses ( t 2 – t 1) > 0 the concentration of reagents decreases, therefore, ( c 2 – c 1) < 0, а т.к. скорость реакции всегда положительна, то перед дробью следует поставить знак «–».
Rice. 1.
|
Quantitatively, the relationship between the reaction rate and the molar concentrations of the reactants is described by the basic law of chemical kinetics - the law of mass action.
The rate of a chemical reaction at a constant temperature is proportional to the product of the concentrations of the reactants.
For reaction
A A+ b B = With C + d D,
in accordance with the law of mass action, the dependence of speed on the concentrations of reacting substances can be presented as:
![]()
Where k– rate constant; n A, n B – reaction orders for reagents A and B, respectively;
n A+ n B is the general reaction order.
In homogeneous reactions, the reacting substances are in the same gas phase or in solution, evenly mixed with each other, the reaction occurs throughout the entire volume of the mixture. The concentration of the reagent is equal to the quotient of the amount of substance divided by the volume of the mixture: With = /V.
Average reaction speed:
![]()
The shorter the time period, the more accurate the reaction rate will be.
Heterogeneous reactions occur at the phase interface: gas - solid, gas - liquid, liquid - solid, solid - solid. Speed reaction
![]()
measured per unit area of contact of reacting substances S.
When considering the thermal effects of chemical reactions, the transformation of reactant molecules (A + B) into product molecules (C + D) is explained from a thermodynamic point of view as “climbing an energy mountain” in the case of endothermic reactions (Fig. 2, A) or “going downhill” for exothermic reactions (Fig. 2, b).
In order for reactant molecules to react, they must first stock up on additional energy in order to overcome the energy barrier on the way to the reaction products. It is significant that such a barrier also exists in the case of exothermic reactions, so that instead of simply “sliding down the hill,” the molecules have to first “climb the hill.”
Rice. 2.
|
The driving force of the reaction is the desire to achieve a minimum of energy.

In order for a reaction to occur, the particles of the reacting substances must collide with each other. As the temperature increases, the number of these collisions increases due to the increase in the kinetic energy of the molecules, therefore the reaction rate increases. But not every collision of molecules of reacting substances leads to their interaction: for molecules to interact, the bonds between the atoms in them must become weaker or break, for which a certain amount of energy must be expended. If the colliding molecules do not have this energy, their collision does not lead to a reaction. The excess energy that molecules must have in order for their collision to lead to the formation of molecules of a new substance is called activation energy this reaction E a, usually measured in J/mol, kJ/mol. Molecules with this energy are called active molecules.
In Fig. 3 shows energy profiles:
a) endothermic reaction, + H = –Q,
N 2 + O 2 2NO – Q;
b) exothermic reaction, – H = +Q,
H 2 + I 2 2HI + Q.
During the reaction, chemical bonds in active molecules weaken and new bonds arise between particles of reacting substances, a transition state is formed - an activated complex, when the old bonds are not completely destroyed, but new ones have already begun to be built. Activation energy is the energy required for the formation of an activated complex. The energy barrier varies; the lower it is, the easier and faster the reaction occurs.
The point located at the top of the energy barrier is called transition state. From this point, the system can freely pass into the reaction product or return to its original state (Fig. 4).
Activation energy is the factor by which the nature of the reactants influences the rate of a reaction. For some reactions it is small, for others it is large. If the activation energy is small (< 40 кДж/моль), то большая часть столкновений между молекулами реагирующих веществ приводит к реакции. Скорость таких реакций велика. Если энергия активации велика (>40 kJ/mol), then in this case only a small part of the collisions of molecules or other particles leads to a reaction. The speed of such a reaction is low.
The reaction rate at a given time can be calculated if you know the number of active collisions of reacting particles per unit time. Therefore, the dependence of the reaction rate on temperature can be written as:
0 exp(– E a/ RT),
where 0 is the reaction rate, provided that each collision leads to interaction ( E a = 0). This expression for the reaction rate is - Arrhenius equation- an important equation in chemical kinetics (for its practical application, see Appendix No. 2, students make reports).
Why do chemical reactions occur at different rates? This is the main question that faces the teacher and the children in the lesson. Students answer it theoretically by conducting laboratory experiments in groups and solving problems.
Group work
The work of the groups includes the following activities:
Experimental study of factors affecting the rate of chemical reactions;
Observation and analysis of the experimental results obtained;
Completing laboratory sheets reflecting progress and conclusions.
A prerequisite for successful work in groups and the implementation of assigned tasks is to provide each student’s workplace with the necessary equipment and visual aids. During work, the teacher approaches all groups and, if necessary, provides advisory assistance. Below is the content of the tasks for each group.
Laboratory experiment No. 1.
Dependence of the rate of a chemical reaction
from the nature of the reactants
Target. Reinforce the concept of “rate of a chemical reaction” and identify its dependence on the nature of the reacting substances.
Equipment and reagents. Rack with test tubes; zinc granules, magnesium tape, hydrochloric acid solution.
Demonstration experience.
Reaction rate and its dependence
on the concentration of starting substances
Target. Visually demonstrate what the rate of a chemical reaction is and how it depends on the concentration of the starting substances.
Equipment and reagents. Computer with measuring unit, optical density sensor at wavelength = 525 nm, cuvette, magnetic stirrer, 5 ml syringe, 100 ml graduated cylinder; solutions - 1M KI, 0.1M K 2 S 2 O 8, distilled water.
Chemical essence of the process. The reaction of oxidation of iodide ion with persulfate is studied:
2I – + S 2 O 8 2– = I 2 + 2SO 4 2– .
The reaction is carried out in excess of potassium iodide. The released iodine turns the solution brown. The iodine concentration is determined by the color intensity of the solution using an optical density sensor at 525 nm.
Preparing for work. An optical density sensor tuned to a wavelength of 525 nm is connected to the first channel of the measuring unit. Turn on the sensor in time-dependent mode, pour 10 ml of 1M KI solution and 90 ml of distilled water into the cuvette. Set up the sensor.
Performance. Start the mixing process. Take 5 ml of K 2 S 2 O 8 solution into a syringe, quickly pour it into the cuvette, simultaneously starting the measurement process by pressing the “Start” screen button. The measurement is stopped when the optical density reaches 0.5.
Repeat the experiment using 20 ml of KI solution and 80 ml of water.
Comments. The rate of a reaction is the change in the concentration of reactants or reaction products per unit time. The rate of the reaction depends on the concentration of the starting reagents at a given time.
Inferred concepts. Reaction rate, its dependence on concentration.
Conclusions. As reactants are consumed during the reaction, the rate slows down.
As the concentration of the starting reagent increases, the reaction rate increases. Moreover, in this case, when the concentration was doubled, the reaction rate also doubled.
Laboratory experiment No. 2.
Effect of temperature on speed
Target. Reinforce the concept of “rate of a chemical reaction” and explore the effect of temperature on the rate of a chemical reaction.
Equipment and reagents. Rack with test tubes, pipette, alcohol lamp, test tube holder; copper(II) oxide, sulfuric acid solution (1:3).
Laboratory experiment No. 3.
Dependence of the rate of a chemical reaction
from the contact surface area
reactants
Target. Reinforce the concept of “rate of a chemical reaction” and identify its dependence on the size of the contact surface of the reacting substances.
Equipment and reagents. Rack with test tubes, glass rod; iron filings, iron nail, copper(II) chloride solution.
Presentation of the results of group work, their discussion
The order in which the results are presented is determined by the group numbers (in turn). Students speak at the board using tables filled in based on the results of their laboratory experiments. A brief discussion of the results of the groups’ work is organized and conclusions are formulated. The teacher points out another factor that affects the rate of a chemical reaction - the presence of a catalyst.
Catalysts- these are substances that speed up a chemical reaction, inhibitors- These are substances that slow down a chemical reaction. The catalysts and inhibitors themselves are not consumed in the reaction and are not included in the reaction products.
Catalysis is the process of changing the rate of a reaction under the influence of a catalyst. The action of the catalyst is selective. Reactions that occur with the participation of a catalyst are called catalytic reactions.
Mechanism of homogeneous catalysis
Often reactions are slow, because... their activation energy E a is large (Fig. 5):
A + B A B AB.
Catalyst (K) speeds up the reaction:

Activation energies E"a and E"" and are small, so the reactions proceed quickly.
With the participation of a catalyst, a decrease occurs E and, a gain in energy is formed and the reaction proceeds faster.
V i d y c a t a l i s a
1. Homogeneous catalysis– starting materials and catalyst – single-phase system.
For example, natural fluctuations in the thickness of the Earth's ozone layer are associated with changes in solar activity. In the upper layers of the atmosphere, the destruction of the ozone layer occurs, catalyzed by nitrogen oxides:

2. Heterogeneous catalysis– the starting materials and the catalyst form a multiphase system.

The mechanism of heterogeneous catalysis includes five stages:
Diffusion - reacting molecules diffuse to the surface of the catalyst;
Adsorption - reactants accumulate on the surface of the catalyst;
Chemical reaction - the surface of the catalyst is heterogeneous, there are active centers on it, they weaken the bonds between atoms in the adsorbed molecules, the reacting molecules are deformed, sometimes break up into atoms, which facilitates the occurrence of a chemical reaction;
Desorption - product molecules are first retained by the surface of the catalyst and then released;
Diffusion - product molecules diffuse from the surface of the catalyst.
Figuratively speaking, the mechanism of action of the catalyst can be compared to tourists crossing a mountain pass. Tourists unfamiliar with the area will choose the most obvious but most difficult route, requiring a long climb and descent over the top of the mountain. An experienced guide (catalyst) will lead his group along the path, past the top. Although this path is winding, it is less difficult; it is easier to reach the final point along it, after which the guide returns to the starting point.
A special group consists of catalysts that act in living organisms. Such catalysts are called enzymes or enzymes.
Enzymes (enzymes)- these are protein molecules that accelerate chemical processes in biological systems (there are about 30 thousand different enzymes in the body, each of them accelerates the corresponding reaction).
Demonstration experience.
Catalytic decomposition of hydrogen peroxide
(conducted by teacher)
2H 2 O 2 2H 2 O + O 2 .
5 ml of a pharmaceutical solution of hydrogen peroxide is poured into three test tubes. The first test tube is a control one; for comparison, a piece of raw meat is dropped into the second test tube with tweezers, and a piece of raw carrot is placed into the third test tube. “Boiling” is observed in two test tubes, except the first. Smoldering splinters are introduced into the second and third test tubes, which flare up because oxygen is released. The teacher explains that the decomposition of hydrogen peroxide occurs without a catalyst, but much more slowly. The reaction may take several months. Rapid reactions in other test tubes demonstrate the work of the enzyme catalase, which is found in both plant and animal cells.
The effectiveness of the catalase enzyme can be illustrated by data on the decomposition of H 2 O 2 in an aqueous solution.
Enzymes are learned in more detail when studying the 11th grade chemistry course.
The development of sustained attention, the ability to observe experience, conduct analysis, and draw conclusions begins with a demonstration experiment. The group form of work allows you to effectively obtain knowledge, fostering a sense of teamwork.
The use of a set of equipment with a computer measuring unit and sensors (temperature, optical density, electrical conductivity, pH level) significantly expands the capabilities of the demonstration experiment, because allows us to look inside the process, which we could not do previously by studying this topic only theoretically. The study of quantitative laws is one of the key and most complex topics in chemistry (see Appendix No. 3 “Parameters used in quantitative chemical calculations”).
In this lesson, we are interested in reaction parameters. In previous lessons, students became familiar with thermodynamic parameters, and the parameters of matter and environment will be studied in subsequent lessons.
Lesson summary, reflective analysis
The teacher sums up the lesson. Students fill out student work control sheets, on which they indicate their class, last name, first name, evaluate their work in the lesson, group work, understanding of the topic (“bad”, “good”, “excellent”).
Students answer questions.
1. In what mood do you leave the lesson?
2. Why is the lesson interesting for each group and each student?
3. What is the benefit of this lesson for you?
4. What difficulties did you encounter in the lesson?
Different classes offer different questions. From experience, we can say that at the reflective stage, students give a high rating to the lesson (“5”, less often “4”), note the unusualness, clarity, richness of the lesson, high emotional level, logic, and interesting information material. The most important technology in a lesson is collaboration between teacher and students. Together, common goals are achieved, students better assimilate the material and apply the acquired knowledge.
Homework
Along with the textbook paragraphs, each group receives an individual task to study the influence of a particular factor on the rate of a chemical reaction.
Task 1. At t= 30 °C the reaction proceeds in 25 minutes, and at t= 50 °C – in 4 minutes. Calculate the temperature coefficient of the reaction.
Task 2. The interaction of aluminum with chlorine proceeds according to the equation:
2Al (solid) + 3Cl 2 (g) = 2AlCl 3 (solid).
The initial concentration of chlorine is 0.05 mol/l. The reaction rate constant is 0.2 l/(mol s).
Write a mathematical expression for the reaction rate. How does the reaction rate change compared to the initial one if the pressure in the system is increased 6 times?
Task 3. In two identical vessels, decomposition reactions were carried out with the formation of oxygen and hydrogen. In 10 s, 22.4 liters of O 2 were obtained in the first vessel, and 4 g of H 2 in the second vessel. Which chemical reaction has the fastest rate? How many times?
Task 4. Suggest ways to increase the reaction rate 16 times by changing the concentrations of the starting substances:
a) 2Cu (solid) + O 2 (g) = 2CuO (solid);
b) 2H 2 (g.) + O 2 (g.) = 2H 2 O (g.).
A special feature of the lesson is that it offers material that goes beyond the scope of the textbook. This is necessary both to increase general erudition and for future applicants. Additional material in the specialized class is based mainly on materials from entrance exams to various universities.
The goal of educational technologies is to increase the efficiency of the educational process. The main thing in any technology is to focus on the student’s personality. Pedagogical technology is a set of interrelated means, methods, and processes necessary for a targeted impact on the formation of a personality with given qualities. I use a student-centered approach in my lessons. As a result, students are able to approach the study of the material more consciously and creatively. It is the technology of cooperation between teacher and student that is important in achieving high results. The active use of elements of pedagogical technologies in the classroom contributes to the development of the student's motivational sphere, intelligence, independence, and the ability to control and manage his educational and cognitive activities.
My subject is chemistry, but I also teach human studies. Using new approaches in education allows you to look at your subject differently. The main thing is to see a person in every student.
Chemistry is the science of substances. I approach the study of substances not only from the point of view of their practical significance for society, but also from the position of a philosophical understanding of the world. In the lessons of chemistry and human studies, I show the integrity of the world and man, I try to reveal to children the infinity and harmony of life, to cultivate the desire to understand and know themselves, the desire to improve themselves, to work on themselves in order to improve their lives. I am pleased with the guys' interest in these problems. And I think it’s useful for us teachers to reflect on this as well. Only by improving and developing ourselves can we teach children.
APPENDIX No. 1
The structure of problem-search activities of teachers and students
on the study of the properties of substances and the essence of chemical reactions
(possible use of information technology)

APPENDIX No. 2
Practical use of the Arrhenius equationExample 1. The speed (frequency) of crickets beeping obeys, although not quite strictly, the Arrhenius equation, gradually increasing in the temperature range from 14.2 °C to 27 °C, with an effective activation energy E a = 51 kJ/mol. Based on the frequency of chirps, you can determine the temperature quite accurately: you need to count their number in 15 seconds and add 40, you get the temperature in degrees Fahrenheit (F) (Americans still use this temperature scale).
So, at 55 F (12.8 °C) the chirping frequency is 1 chirp/s, and at 100 F (37.8 °C) - 4 chirps/s.
Example 2.
In the temperature range from 18 °C to 34 °C, the sea turtle's heart rate is consistent with the Arrhenius equation, which gives the activation energy
E a = 76.6 kJ/mol, but at lower temperatures the activation energy increases sharply. This may be due to the fact that at low temperatures the turtle does not feel very well and its heart rate begins to be controlled by other biochemical reactions.
Example 3.
Particularly interesting are attempts to “put Arrhenius dependence” on human psychological processes. Thus, people with different body temperatures (from 36.4 °C to 39 °C) were asked to count the seconds. It turned out that the higher the temperature, the faster the counting.
(E a = 100.4 kJ/mol). Thus, our subjective sense of time obeys the Arrhenius equation. The author of the sociological study, G. Hogland, suggested that this is due to certain biochemical processes in the human brain.
The German researcher H. von Foerstler measured the rate of forgetting in people with different temperatures. He gave people a sequence of different signs and measured the time during which people remembered this sequence. The result was the same as Hoagland's: Arrhenius dependence with E a = 100.4 kJ/mol.
These examples show that many processes in nature, including psychological ones, obey the Arrhenius equation with fairly high activation energies E A. This last point is especially important because E and physical processes (for example, viscous fluid flow) usually do not exceed 20 kJ/mol. A high activation energy usually means that chemical bonds are broken. So in all the examples discussed, there are undoubtedly real chemical reactions taking place (obviously enzymatic).
APPENDIX No. 3
The equilibrium of the process of transition from one phase to another without changing the chemical composition is called phase equilibrium. Examples of phase equilibrium can be the following processes:
evaporation
![]()
For phase equilibrium, Le Chatelier's principle is observed.
As the temperature increases, the equilibrium shifts towards an endothermic process, such as melting and evaporation. With increasing pressure, the equilibrium shifts towards processes in which gas or vapor turns into a liquid or solid state.
Phase rule.
Formulated by J. Gibbs. Number of degrees of freedom C , phases F , independent components TO and external conditions n , affecting balance, are interconnected by the relationship:
C+ F = K + n
2.4. Mechanism of chemical reactions. Chain reactions. Photochemical processes. Homogeneous and heterogeneous catalysis. Autocatalysis. Enzyme catalysis. Catalytic poisons. Oscillatory reactions.
Activation energy. Energy profile of the reaction.
Reactions occur as a result of direct collisions of molecules. However, not all collisions result in a chemical reaction. The formation of new substances is facilitated only by molecules that have a sufficient supply of energy. Such molecules are called active molecules.
The minimum energy sufficient to start a chemical reaction is called activation energy and is expressed in kcal or kJ. The lower the activation energy, the faster the reaction occurs.
In reactions where the activation energy is greater than 150 kJ at t=25°C, the rate is very low or practically these reactions do not occur. In reactions where the activation energy is less than 60 kJ, the speed is very high (explosion).
The activation energy Ea depends on the nature of the reacting elements and serves as a characteristic of each reaction.

Energy diagram of the reaction with the formation
activated complex.
In order for reactants A and B to form reaction products C and D, they must overcome the energy barrier ML. This requires activation energy Ea. In this case, during the reaction, an intermediate unstable group is formed from particles of the implementing substances - an activated complex (Fig. 2.6).
This complex decomposes to form the final products, and such an amount of energy is released that allows the final products to descend to the level of the average energy of the final products.
That. the change in products can be expressed in the form of diagrams for endothermic and exothermic reactions (Fig. 2.7, 2.8).


flow diagram
exothermic reaction
flow diagram
endothermic reaction
ABOUT  Typically, reactions between substances with strong covalent bonds are characterized by large Ea values and proceed slowly. This applies to many interactions, like
Typically, reactions between substances with strong covalent bonds are characterized by large Ea values and proceed slowly. This applies to many interactions, like
whose speed under standard conditions is 0.
M  ionic interactions in solutions are characterized by low Ea values and very high velocities
ionic interactions in solutions are characterized by low Ea values and very high velocities
Energy profile of the reaction. A + B = AB (without catalyst) A + B + K? + B? ? AB + K (with cat.).
Picture 45 from the presentation “Rate of chemical reaction” for chemistry lessons on the topic “Reactions”Dimensions: 1280 x 800 pixels, format: jpg. To download a free picture for a chemistry lesson, right-click on the image and click “Save image as...”. To display pictures in the lesson, you can also download for free the entire presentation “Rate of a chemical reaction.ppt” with all the pictures in a zip archive. The archive size is 129 KB.
Download presentationReactions
“Chemical Equations” - 7 Н2SO4. Law of conservation of mass of substances. Ca + O2 CaO. Topic: Changes occurring in substances. Signs and conditions for the occurrence of chemical reactions. REMEMBER! Chemical equations. Modern wording of the law: 1756
“Electrolytic dissociation of salts” - Application of salts. Phenolphthalein solution Write down the molecular and ionic equations for the possible reactions. Chemical properties of salts. 1. Metal + salt 2. Salt + alkali 3. Salt + acid 4. Salt + salt. Task 3. Which of the following substances does a sodium hydroxide solution react with? NaOH, Ba(OH)2, NH4OH, Al(OH)3.
“Equations of chemical reactions” - D/Z 1) study the text § 26 2) complete written exercises No. 1-3. Setting a goal. 2) View student presentations on water purification at home. Production of carbon dioxide by the interaction of soda and acid. Hydrogen atom. 4. View student presentations on selected topics. m1. Reference material for group work.
“Rate of chemical reaction” - t1. dCB dt. The rate of a chemical reaction. a A. Chemical kinetics. dc dt. Classification of processes by phase composition. V a) n=0 v b) n=1 v c) n>1. Chain - unbranched districts. C1. Graphic definition of n. Lecture plan. Chain - branched reactions. Kinetic equation of a complex reaction.
“Reactions of substances” - Classification of substances by composition: Photos of fragments of lessons using an interactive whiteboard. N2. Grade 10 “Carbohydrates”. What substances are discussed in the excerpt from S. Shchipachev’s poem “Reading Mendeleev”? Write the reaction equations for the production of aluminum sulfate. Task No. 4. Task No. 7. Cinnabar mercury (ii) sulfide.
“Types of chemical reactions” - All reactions are accompanied by thermal effects. Types of chemical reactions. Chemical reactions occur: when mixing or physical contact of reagents, spontaneously when heated with the participation of catalysts, the action of light, electric current, mechanical action, etc. Karpukhina Irina Stepanovna Chemistry teacher MBOU Secondary School No. 32 City of Novosibirsk.
There are 28 presentations in total
The enthalpy change DH is essentially the difference in the bond energies of the reactants and products, including the energies of conjugation, tension, and solvation. DH can be calculated by summing the energies of all bonds broken during the reaction and subtracting from them the sum of the energies of all bonds formed, adding all changes in conjugation, tension and solvation energies. In addition, the change in enthalpy can be determined experimentally by measuring the thermal effect of the reaction, since the change in enthalpy is equal to the thermal effect of the reaction, taken with the opposite sign.
- DH=Qр
The change in entropy DS characterizes the degree of disorder of the system. In organic chemistry, this factor rarely plays a big role, because reactions occur at relatively low temperatures, at which the entropy factor is small. However, in some cases, entropy change can play a significant role:
· since gases have higher entropy than liquids (even more so than solids), then any reaction in which the starting substances are liquid or solid, and one or more products are gaseous, is thermodynamically favorable, since the entropy of the system increases;
· if during a reaction more molecules of products are formed than molecules of starting substances, then the reaction proceeds with an increase in entropy.
A negative DG value in itself does not mean that the reaction will occur in the foreseeable period of time. A negative change in free energy is a necessary but not sufficient factor for the spontaneous occurrence of a chemical reaction. For example, the reaction of two moles of hydrogen with one mole of oxygen, resulting in the formation of water, is characterized by a large negative change in free energy. However, a mixture of O 2 and H 2 can be stored at room temperature for decades without any sign of a chemical reaction.
Mechanisms of organic reactions
To understand organic reactions, knowledge of their mechanisms is extremely useful.
Reaction mechanism - a detailed description of the process of converting starting compounds into products. The mechanism includes data on the method and sequence of bond cleavage and formation, the structure of intermediates (intermediate products), kinetics, thermodynamics and stereochemistry of the reaction. The mechanism should not contradict existing experimental facts, and when new ones appear, explain them too.
When considering the subtle features of mechanisms, it is extremely useful to use the so-called energy diagram (energy profile) reactions. This is a graphical dependence of the energy of the system on a complex function of the distance between reacting substances, which is usually called " reaction coordinate" or " progress of the reaction"(Figure 3.1).
Rice. 3.1. Energy diagram: A – endo-, B – exothermic reaction.
This figure illustrates the occurrence of one-step reactions. An endothermic reaction involves the absorption of heat, while an exothermic reaction involves the release of heat.
Almost all chemical reactions occur when two or more, very rarely, reacting particles collide. From Fig. 3.1 it is clear that the approach of reacting molecules leads to an increase in the energy of the system to a certain maximum. Collisions will be effective when the reacting substances have some excess energy compared to the average energy of the molecules in the system. Particles that do not have such an excess of energy scatter in different directions after a collision. Activation energy- excess energy required to overcome the energy barrier. The maximum energy of the system (the highest point of the energy diagram) corresponds to transition state (activated complex). It is the presence of a transition state that explains the reason that even exothermic reactions usually do not occur spontaneously, but only upon heating or other means of activating the system.
It is the transition state - the highest energy point of the reaction - that determines the course of the entire transformation. Knowledge of its structure can clarify the mechanism of chemical transformation. However, the lifetime of the activated complex is so short that there are no physical methods to register it and, consequently, to obtain knowledge about its structure.
J. Hammond's postulate
To indirectly assess the structure of the transition state, use postulate of J. Hammond (1955): minor energy changes are accompanied by minor changes in molecular structure. More clear wording: the structure of the transition state is similar to the structure of those substances to which it is closer in energy. In exothermic reactions, the transition state is closer in structure to the initial reagents (Fig. 3.1). This activated complex is called early transitional state. The transition state in endothermic reactions is closer in structure to the reaction products; it is called late. Similar impacts on similar structures lead to similar results. Therefore everything factors stabilizing(lowering energy states) energetically close to the transition state initial, intermediate or final substance, reduce the energy of the activated complex.
The use of Hammond's postulate is especially useful when considering multi-step reactions (Figure 3.2).
 |
Figure 3.2. Energy diagram of a two-step reaction
From Figure 3.2 it is clear that the reaction occurs in two stages, through one intermediate product. The conversion of products into intermediates (first stage) is more important for the overall reaction than the conversion of intermediates into reaction products (second stage). This is confirmed by the corresponding activation energies of the first and second stages (Ea 1 and Ea 2, respectively). The entire course of a reaction is determined by its highest energy point - the transition state of the first stage [PS 1]. If we apply Hammond's postulate to this reaction, it is easy to conclude that the intermediate product is energetically closest to the transition states of both stages of the reaction.
S. Arrhenius discovered the temperature dependence of the rate of many reactions, which can be described by the equation:
k = A e - E* / RT
Where k - rate constant, e - base of natural logarithms, R - universal gas constant, T - temperature, A - pre-exponential factor, E* - reaction activation energy.
active collision theory(collisions)
1) Chemical interaction between molecules is possible only when they collide.
2) Not every collision of molecules leads to a chemical interaction, that is, it is effective or, in Arrhenius’ terminology, active. There is a certain energy barrier, which only a portion of the molecules can overcome and interact with, and, as a rule, this is a very small portion of their total number in the system.
3) The reason for the existence of an energy barrier is the mutual repulsion of the electron shells of molecules when they approach each other.
4) In order for molecules to overcome the energy barrier during a collision, they must move towards each other at a sufficiently high speed. To achieve this required speed, a certain energy is needed, called activation energy. Activation energy E* - this is the excess energy of active molecules compared to inactive ones, or otherwise, the energy that molecules must have in order to be able to interact. The SI dimension of activation energy is J/mol.
5) The greater the activation energy of the reaction, the greater the energy barrier, and the fewer molecules are able to overcome it. Therefore, the more E* , the slower the reaction goes.
6) With increasing temperature, the speed of thermal movement of molecules increases, so the proportion of active molecules increases. In other words, as the temperature increases, thermal activation, leading to an increase in the reaction rate.
calculating the activation energy of a reaction from the values of two rate constants at different temperatures:
T 2 - T 1 k 1
Activated complex is an unstable formation that includes all the atoms of colliding and interacting molecules. The lifetime of the activated complex is very short; it is measured in small (millionths, ten-millionths, etc.) fractions of a second. The distances between atoms in an activated complex are somewhat greater than in ordinary molecules, so additional energy is required for its formation.
A visual representation of the course of a reaction over time in accordance with the transition state theory can give energy profile reaction, for example, exothermic (Fig. 12.6).
The energy of the system is plotted along the ordinate axis E , and the x-axis is the so-called reaction coordinate. The average energy reserve of thermal motion of the molecules of the starting substances corresponds to the level E ref, energy stored in the activated complex - level E AK. Then the difference E AK - E ref is equal to the value of the energy barrier that molecules must overcome in order to interact (activation energy). A visual representation of it is given by the curve connecting the levels E ref and E AK. The height of the energy barrier depends on the nature of the reacting substances, the energy required for the formation of the activated complex (activation energy), as well as on the average energy of thermal motion of the molecules E ref.
As the temperature rises, the level E ref rises, the energy barrier becomes smaller and a larger number of molecules can interact. This is the reason why the reaction accelerates with increasing temperature. When the temperature decreases, on the contrary, the level E ref decreases and the energy barrier increases, which leads to a decrease in the reaction rate.
During the decomposition of the activated complex with the formation of product molecules, energy is released, which corresponds to the difference E AK - E prod, where E prod - the average energy reserve of product molecules. Part of this released energy equal to the difference E AK - E ref will be used to activate new molecules of the starting substances, and the excess E ref - E product will be released into the environment in the form of an exothermic thermal effect of the reaction DH r .
For endothermic reactions, the energy profile looks slightly different (Fig. 12.7). It can be seen that in this case the energy level E ref lower than level E cont. As a result of this energy E AK - E the product released during the disintegration of the activated complex is not enough to
to cause the activation of new reactant molecules. Therefore, to continue the reaction, it is necessary to supply energy from the outside, in the form of an endothermic thermal effect.
The existence of an activated complex is confirmed by experimental data. So, for example, for one of the simple model reactions of interaction of a hydrogen atom with a hydrogen molecule
Н 2 + Н ® Н + Н 2 ,
the activation energy value is close to 36.8 kJ/mol. If the reaction proceeded through the stage of complete dissociation of H 2 molecules, and not through the stage of formation of the activated complex H 2 ·H, then an activation energy of 435.1 kJ/mol would be required.
55. photochemistry. Photochemical reactions. Basic laws of photochemistry (Grotthus-Draper law, Bunsen-Roscoe law, Stark-Einstein law).
photochemistry this is a branch of chemical kinetics that deals with the behavior of electronically excited molecules .
In biology, several photochemical processes are known that are extremely important for the life of both individual organisms and the biosphere as a whole. First of all, among such processes we should name photosynthesis In addition, the photochemistry of vision, the photochemistry of vitamin synthesis, for example, vitamin D in human skin, the photochemistry of tanning, etc. are extremely important.
The photochemical reaction of decomposition of silver halides underlies the photographic process. There are photochromic materials that can change color or transparency when exposed to light, which are used, in particular, for photochemical recording of information or for the manufacture of sunglasses. Photochemical reactions are also used in the chemical industry, for example, in the synthesis of caprolactam or in the photopolymerization of methyl methacrylate in the production of organic glass.
For pharmacy, photochemical reactions are important primarily insofar as light can cause destruction ( photolysis)many medications. Many other substances and materials are also subject to decomposition under the influence of light - wood, paper, paints, plastics, etc.
In chemistry, the luminescent method of analysis is widely used, based on the study of the spectra of radiation emitted by excited molecules of the substances under study.
Laws of photochemistry
The following laws of photochemistry are known:
Grotthus-Draper law(K.I.D. Grotthus - 1818; J.W. Draper - 1842).