Millions of biochemical reactions take place in any cell of our body. They are catalyzed by a variety of enzymes, which often require energy. Where does the cell get it? This question can be answered if we consider the structure of the ATP molecule - one of the main sources of energy.
ATP is a universal energy source
ATP stands for adenosine triphosphate, or adenosine triphosphate. The substance is one of the two most important sources of energy in any cell. The structure of ATP and its biological role are closely related. Most biochemical reactions can occur only with the participation of molecules of a substance, this is especially true. However, ATP is rarely directly involved in the reaction: for any process to occur, the energy contained precisely in adenosine triphosphate is needed.
The structure of the molecules of the substance is such that the bonds formed between phosphate groups carry a huge amount of energy. Therefore, such bonds are also called macroergic, or macroenergetic (macro=many, large amount). The term was first introduced by the scientist F. Lipman, and he also proposed using the symbol ̴ to designate them.
It is very important for the cell to maintain a constant level of adenosine triphosphate. This is especially true for muscle tissue cells and nerve fibers, because they are the most energy-dependent and require a high content of adenosine triphosphate to perform their functions.
The structure of the ATP molecule
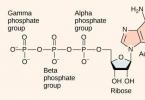
Adenosine triphosphate consists of three elements: ribose, adenine and residues

Ribose- a carbohydrate that belongs to the pentose group. This means that ribose contains 5 carbon atoms, which are enclosed in a cycle. Ribose connects to adenine through a β-N-glycosidic bond on the 1st carbon atom. Phosphoric acid residues on the 5th carbon atom are also added to the pentose.
Adenine is a nitrogenous base. Depending on which nitrogenous base is attached to ribose, GTP (guanosine triphosphate), TTP (thymidine triphosphate), CTP (cytidine triphosphate) and UTP (uridine triphosphate) are also distinguished. All these substances are similar in structure to adenosine triphosphate and perform approximately the same functions, but they are much less common in the cell.
Phosphoric acid residues. A maximum of three phosphoric acid residues can be attached to ribose. If there are two or only one, then the substance is called ADP (diphosphate) or AMP (monophosphate). It is between the phosphorus residues that macroenergetic bonds are concluded, after the rupture of which 40 to 60 kJ of energy is released. If two bonds are broken, 80, less often - 120 kJ of energy is released. When the bond between ribose and the phosphorus residue is broken, only 13.8 kJ is released, so there are only two high-energy bonds in the triphosphate molecule (P ̴ P ̴ P), and in the ADP molecule there is one (P ̴ P).
These are the structural features of ATP. Due to the fact that a macroenergetic bond is formed between phosphoric acid residues, the structure and functions of ATP are interconnected.
The structure of ATP and the biological role of the molecule. Additional functions of adenosine triphosphate
In addition to energy, ATP can perform many other functions in the cell. Along with other nucleotide triphosphates, triphosphate is involved in the construction of nucleic acids. In this case, ATP, GTP, TTP, CTP and UTP are suppliers of nitrogenous bases. This property is used in processes and transcription.
ATP is also necessary for the functioning of ion channels. For example, the Na-K channel pumps 3 sodium molecules out of the cell and pumps 2 potassium molecules into the cell. This ion current is needed to maintain a positive charge on the outer surface of the membrane, and only with the help of adenosine triphosphate can the channel function. The same applies to proton and calcium channels.
ATP is the precursor of the second messenger cAMP (cyclic adenosine monophosphate) - cAMP not only transmits the signal received by cell membrane receptors, but is also an allosteric effector. Allosteric effectors are substances that speed up or slow down enzymatic reactions. Thus, cyclic adenosine triphosphate inhibits the synthesis of an enzyme that catalyzes the breakdown of lactose in bacterial cells.
The adenosine triphosphate molecule itself may also be an allosteric effector. Moreover, in such processes, ADP acts as an antagonist to ATP: if triphosphate accelerates the reaction, then diphosphate inhibits it, and vice versa. These are the functions and structure of ATP.

How is ATP formed in a cell?
The functions and structure of ATP are such that the molecules of the substance are quickly used and destroyed. Therefore, triphosphate synthesis is an important process in the formation of energy in the cell.
There are three most important methods for the synthesis of adenosine triphosphate:
1. Substrate phosphorylation.
2. Oxidative phosphorylation.
3. Photophosphorylation.
Substrate phosphorylation is based on multiple reactions occurring in the cell cytoplasm. These reactions are called glycolysis - anaerobic stage. As a result of 1 cycle of glycolysis, from 1 molecule of glucose two molecules are synthesized, which are then used to produce energy, and two ATP are also synthesized.
- C 6 H 12 O 6 + 2ADP + 2Pn --> 2C 3 H 4 O 3 + 2ATP + 4H.
Cell respiration
Oxidative phosphorylation is the formation of adenosine triphosphate by transferring electrons along the membrane electron transport chain. As a result of this transfer, a proton gradient is formed on one side of the membrane and, with the help of the protein integral set of ATP synthase, molecules are built. The process takes place on the mitochondrial membrane.

The sequence of stages of glycolysis and oxidative phosphorylation in mitochondria constitutes a common process called respiration. After a complete cycle, 36 ATP molecules are formed from 1 glucose molecule in the cell.
Photophosphorylation
The process of photophosphorylation is the same as oxidative phosphorylation with only one difference: photophosphorylation reactions occur in the chloroplasts of the cell under the influence of light. ATP is produced during the light stage of photosynthesis, the main energy production process in green plants, algae and some bacteria.
During photosynthesis, electrons pass through the same electron transport chain, resulting in the formation of a proton gradient. The concentration of protons on one side of the membrane is the source of ATP synthesis. The assembly of molecules is carried out by the enzyme ATP synthase.

The average cell contains 0.04% adenosine triphosphate by weight. However, the highest value is observed in muscle cells: 0.2-0.5%.
There are about 1 billion ATP molecules in a cell.
Each molecule lives no more than 1 minute.
One molecule of adenosine triphosphate is renewed 2000-3000 times a day.
In total, the human body synthesizes 40 kg of adenosine triphosphate per day, and at any given time the ATP reserve is 250 g.

Conclusion
The structure of ATP and the biological role of its molecules are closely related. The substance plays a key role in life processes, because the high-energy bonds between phosphate residues contain a huge amount of energy. Adenosine triphosphate performs many functions in the cell, and therefore it is important to maintain a constant concentration of the substance. Decay and synthesis occur at high speed, since the energy of bonds is constantly used in biochemical reactions. This is an essential substance for any cell in the body. That's probably all that can be said about the structure of ATP.
ATP is the abbreviation for Adenosine Tri-Phosphoric Acid. You can also find the name Adenosine triphosphate. This is a nucleoid that plays a huge role in energy exchange in the body. Adenosine Tri-Phosphoric acid is a universal source of energy involved in all biochemical processes of the body. This molecule was discovered in 1929 by the scientist Karl Lohmann. And its significance was confirmed by Fritz Lipmann in 1941.
Structure and formula of ATP
If we talk about ATP in more detail, then this is a molecule that provides energy to all processes occurring in the body, including the energy for movement. When the ATP molecule is broken down, the muscle fiber contracts, resulting in the release of energy that allows contraction to occur. Adenosine triphosphate is synthesized from inosine in a living organism.
In order to give the body energy, adenosine triphosphate must go through several stages. First, one of the phosphates is separated using a special coenzyme. Each phosphate provides ten calories. The process produces energy and produces ADP (adenosine diphosphate).
If the body needs more energy to function, then another phosphate is separated. Then AMP (adenosine monophosphate) is formed. The main source for the production of Adenosine Triphosphate is glucose; in the cell it is broken down into pyruvate and cytosol. Adenosine triphosphate energizes long fibers that contain the protein myosin. It is what forms muscle cells.
At moments when the body is resting, the chain goes in the opposite direction, i.e. Adenosine Tri-Phosphoric acid is formed. Again, glucose is used for these purposes. The created Adenosine Triphosphate molecules will be reused as soon as necessary. When energy is not needed, it is stored in the body and released as soon as it is needed.
The ATP molecule consists of several, or rather, three components:
- Ribose is a five-carbon sugar that forms the basis of DNA.
- Adenine is the combined atoms of nitrogen and carbon.
- Triphosphate.
At the very center of the adenosine triphosphate molecule is a ribose molecule, and its edge is the main one for adenosine. On the other side of ribose is a chain of three phosphates.
ATP systems
 At the same time, you need to understand that ATP reserves will be sufficient only for the first two or three seconds of physical activity, after which its level decreases. But at the same time, muscle work can only be carried out with the help of ATP. Thanks to special systems in the body, new ATP molecules are constantly synthesized. The inclusion of new molecules occurs depending on the duration of the load.
At the same time, you need to understand that ATP reserves will be sufficient only for the first two or three seconds of physical activity, after which its level decreases. But at the same time, muscle work can only be carried out with the help of ATP. Thanks to special systems in the body, new ATP molecules are constantly synthesized. The inclusion of new molecules occurs depending on the duration of the load.
ATP molecules synthesize three main biochemical systems:
- Phosphagen system (creatine phosphate).
- Glycogen and lactic acid system.
- Aerobic respiration.
Let's consider each of them separately.
Phosphagen system- if the muscles work for a short time, but extremely intensely (about 10 seconds), the phosphagen system will be used. In this case, ADP binds to creatine phosphate. Thanks to this system, a small amount of Adenosine Triphosphate is constantly circulated in muscle cells. Since the muscle cells themselves also contain creatine phosphate, it is used to restore ATP levels after high-intensity short work. But within ten seconds the level of creatine phosphate begins to decrease - this energy is enough for a short race or intense strength training in bodybuilding.
 Glycogen and lactic acid- supplies energy to the body more slowly than the previous one. It synthesizes ATP, which can be enough for one and a half minutes of intense work. In the process, glucose in muscle cells is formed into lactic acid through anaerobic metabolism.
Glycogen and lactic acid- supplies energy to the body more slowly than the previous one. It synthesizes ATP, which can be enough for one and a half minutes of intense work. In the process, glucose in muscle cells is formed into lactic acid through anaerobic metabolism.
Since in the anaerobic state oxygen is not used by the body, this system provides energy in the same way as in the aerobic system, but time is saved. In anaerobic mode, muscles contract extremely powerfully and quickly. Such a system can allow you to run a four hundred meter sprint or a longer intense workout in the gym. But working in this way for a long time will not allow muscle soreness, which appears due to an excess of lactic acid.
Aerobic respiration- this system turns on if the workout lasts more than two minutes. Then the muscles begin to receive adenosine triphosphate from carbohydrates, fats and proteins. In this case, ATP is synthesized slowly, but the energy lasts for a long time - physical activity can last for several hours. This happens due to the fact that glucose breaks down without obstacles, it does not have any counteractions from outside - as lactic acid interferes with the anaerobic process.
The role of ATP in the body
From the previous description it is clear that the main role of adenosine triphosphate in the body is to provide energy for all the numerous biochemical processes and reactions in the body. Most energy-consuming processes in living beings occur thanks to ATP.
But in addition to this main function, adenosine triphosphate also performs others:

The role of ATP in the human body and life is well known not only to scientists, but also to many athletes and bodybuilders, since its understanding helps make training more effective and correctly calculate loads. For people who do strength training in the gym, sprinting and other sports, it is very important to understand what exercises need to be performed at one time or another. Thanks to this, you can form the desired body structure, work out the muscle structure, reduce excess weight and achieve other desired results.
Metabolic processes include reactions that consume energy and reactions that release energy. In some cases, these reactions are coupled. However, often the reactions in which energy is released are separated in space and time from the reactions in which it is consumed. In the process of evolution, plant and animal organisms have developed the ability to store energy in the form of compounds that have rich energy bonds. Among them, adenosine triphosphate (ATP) occupies a central place. ATP is a nucleotide phosphate consisting of a nitrogenous base (adenine), a pentose (ribose) and three molecules of phosphoric acid. The two terminal molecules of phosphoric acid form high-energy, energy-rich bonds. ATP is contained in the cell mainly in the form of a complex with magnesium ions. During respiration, adenosine triphosphate is formed from adenosine diphosphate and the remainder of inorganic phosphoric acid (Pn) using energy released during the oxidation of various organic substances:
ADP + FN --> ATP + H2O
In this case, the oxidation energy of organic compounds is converted into phosphorus bond energy.
In 1939--1940 F. Lipman established that ATP serves as the main carrier of energy in the cell. The special properties of this substance are determined by the fact that the terminal phosphate group is easily transferred from ATP to other compounds or is cleaved off, releasing energy that can be used for physiological functions. This energy is the difference between the free energy of ATP and the free energy of the resulting products (AG). AG is the change in free energy of a system or the amount of excess energy that is released when chemical bonds are reorganized. The breakdown of ATP occurs according to the equation ATP + H20 = ADP + FN, in which case the battery is discharged, and at pH 7 AG = -30.6 kJ is released. This process is catalyzed by the enzyme adenosine triphosphatase (ATPase). The equilibrium of ATP hydrolysis is shifted towards the completion of the reaction, which determines the large negative value of the free energy of hydrolysis. This is due to the fact that during dissociation. With four hydroxyl groups at pH 7, ATP has four negative charges. The close arrangement of charges to each other promotes their repulsion and, consequently, the detachment of phosphate groups. As a result of hydrolysis, compounds with the same charge are formed (ADP3~ and HP04~), which become independent of each other, which prevents their connection. The unique properties of ATP are explained not only by the fact that during its hydrolysis a large amount of energy is released, but also by the fact that it has the ability to donate the terminal phosphate group along with the energy reserve to other organic compounds. The energy contained in the macroergic phosphorus bond is used for the physiological activity of the cell. At the same time, in terms of the free energy of hydrolysis - 30.6 kJ/mol, ATP occupies an intermediate position. Thanks to this, the ATP-ADP system can serve as a carrier of phosphate groups from phosphorus compounds with higher hydrolysis energy, for example phosphoenolpyruvate (53.6 K/mol), to compounds with lower hydrolysis energy, for example sugar phosphates (13.8 kJ/mol) . Thus, the ADF system is, as it were, intermediate or conjugating.
Mechanism of ATP synthesis. The diffusion of protons back through the inner membrane of the mitochondrion is coupled with the synthesis of ATP using the ATPase complex, called coupling factor F,. On electron microscopic images, these factors appear as globular mushroom-shaped formations on the inner membrane of mitochondria, with their “heads” protruding into the matrix. F 1 is a water-soluble protein consisting of 9 subunits of five different types. The protein is an ATPase and is associated with the membrane through another protein complex F0, which laces the membrane. F 0 does not exhibit catalytic activity, but serves as a channel for the transport of H + ions through the membrane to Fx.
The mechanism of ATP synthesis in the Fi~ F 0 complex is not fully understood. There are a number of hypotheses on this score.
One of the hypotheses explaining the formation of ATP through the so-called direct mechanism, was suggested by Mitchell.
Rice. 9. Possible mechanisms of ATP formation in the F 1 - F 0 complex
According to this scheme, at the first stage of phosphorylation, the phosphate ion and ADP bind to the g component of the enzyme complex (A). Protons move through the channel in the F 0 component and combine in the phosphate with one of the oxygen atoms, which is removed as a water molecule (B). The oxygen atom of ADP combines with a phosphorus atom to form ATP, after which the ATP molecule is separated from the enzyme (B).
For indirect mechanism Various options are possible. ADP and inorganic phosphate are added to the active site of the enzyme without an influx of free energy. H + ions, moving along the proton channel along the gradient of their electrochemical potential, bind in certain areas of F b causing conformational changes. changes in the enzyme (P. Boyer), as a result of which ATP is synthesized from ADP and P i. The release of protons into the matrix is accompanied by the return of the ATP synthetase complex to its original conformational state and the release of ATP.
In energized form, F 1 functions as an ATP synthetase. In the absence of coupling between the electrochemical potential of H + ions and ATP synthesis, the energy released as a result of the reverse transport of H + ions in the matrix can be converted into heat. Sometimes this is beneficial, since increasing the temperature in the cells activates the enzymes.
Continuation. See No. 11, 12, 13, 14, 15, 16/2005
Biology lessons in science classes
Advanced planning, grade 10
Lesson 19. Chemical structure and biological role of ATP
Equipment: tables on general biology, diagram of the structure of the ATP molecule, diagram of the relationship between plastic and energy metabolism.
I. Test of knowledge
Conducting a biological dictation “Organic compounds of living matter”
The teacher reads the abstracts under numbers, the students write down in their notebooks the numbers of those abstracts that match the content of their version.
Option 1 – proteins.
Option 2 – carbohydrates.
Option 3 – lipids.
Option 4 – nucleic acids.
1. In their pure form they consist only of C, H, O atoms.
2. In addition to C, H, O atoms, they contain N and usually S atoms.
3. In addition to C, H, O atoms, they contain N and P atoms.
4. They have a relatively small molecular weight.
5. The molecular weight can be from thousands to several tens and hundreds of thousands of daltons.
6. The largest organic compounds with a molecular weight of up to several tens and hundreds of millions of daltons.
7. They have different molecular weights - from very small to very high, depending on whether the substance is a monomer or a polymer.
8. Consist of monosaccharides.
9. Consist of amino acids.
10. Consist of nucleotides.
11. They are esters of higher fatty acids.
12. Basic structural unit: “nitrogen base–pentose–phosphoric acid residue.”
13. Basic structural unit: “amino acids”.
14. Basic structural unit: “monosaccharide”.
15. Basic structural unit: “glycerol–fatty acid.”
16. Polymer molecules are built from identical monomers.
17. Polymer molecules are built from similar, but not quite identical monomers.
18. They are not polymers.
19. They perform almost exclusively energy, construction and storage functions, and in some cases – protective.
20. In addition to energy and construction, they perform catalytic, signaling, transport, motor and protective functions;
21. They store and transmit the hereditary properties of the cell and organism.
Option 1 – 2; 5; 9; 13; 17; 20.
Option 2 – 1; 7; 8; 14; 16; 19.
Option 3 – 1; 4; 11; 15; 18; 19.
Option 4– 3; 6; 10; 12; 17; 21.
II. Learning new material
1. Structure of adenosine triphosphoric acid
In addition to proteins, nucleic acids, fats and carbohydrates, a large number of other organic compounds are synthesized in living matter. Among them, an important role is played in the bioenergetics of the cell. adenosine triphosphoric acid (ATP). ATP is found in all plant and animal cells. In cells, adenosine triphosphoric acid is most often present in the form of salts called adenosine triphosphates. The amount of ATP fluctuates and averages 0.04% (on average there are about 1 billion ATP molecules in a cell). The largest amount of ATP is contained in skeletal muscles (0.2–0.5%).
The ATP molecule consists of a nitrogenous base - adenine, a pentose - ribose and three phosphoric acid residues, i.e. ATP is a special adenyl nucleotide. Unlike other nucleotides, ATP contains not one, but three phosphoric acid residues. ATP refers to macroergic substances - substances containing a large amount of energy in their bonds.
Spatial model (A) and structural formula (B) of the ATP molecule
The phosphoric acid residue is cleaved from ATP under the action of ATPase enzymes. ATP has a strong tendency to detach its terminal phosphate group:
ATP 4– + H 2 O ––> ADP 3– + 30.5 kJ + Fn,
because this leads to the disappearance of the energetically unfavorable electrostatic repulsion between adjacent negative charges. The resulting phosphate is stabilized due to the formation of energetically favorable hydrogen bonds with water. The charge distribution in the ADP + Fn system becomes more stable than in ATP. This reaction releases 30.5 kJ (breaking a normal covalent bond releases 12 kJ).
In order to emphasize the high energy “cost” of the phosphorus-oxygen bond in ATP, it is usually denoted by the sign ~ and called a macroenergetic bond. When one molecule of phosphoric acid is removed, ATP is converted to ADP (adenosine diphosphoric acid), and if two molecules of phosphoric acid are removed, ATP is converted to AMP (adenosine monophosphoric acid). The cleavage of the third phosphate is accompanied by the release of only 13.8 kJ, so that there are only two actual high-energy bonds in the ATP molecule.
2. ATP formation in the cell
The supply of ATP in the cell is small. For example, ATP reserves in a muscle are enough for 20–30 contractions. But a muscle can work for hours and produce thousands of contractions. Therefore, along with the breakdown of ATP to ADP, reverse synthesis must continuously occur in the cell. There are several pathways for ATP synthesis in cells. Let's get to know them.
1. Anaerobic phosphorylation. Phosphorylation is the process of ATP synthesis from ADP and low molecular weight phosphate (Pn). In this case, we are talking about oxygen-free processes of oxidation of organic substances (for example, glycolysis is the process of oxygen-free oxidation of glucose to pyruvic acid). Approximately 40% of the energy released during these processes (about 200 kJ/mol glucose) is spent on ATP synthesis, and the rest is dissipated as heat:
C 6 H 12 O 6 + 2ADP + 2Pn ––> 2C 3 H 4 O 3 + 2ATP + 4H.
2. Oxidative phosphorylation is the process of ATP synthesis using the energy of oxidation of organic substances with oxygen. This process was discovered in the early 1930s. XX century V.A. Engelhardt. Oxygen processes of oxidation of organic substances occur in mitochondria. Approximately 55% of the energy released in this case (about 2600 kJ/mol glucose) is converted into the energy of chemical bonds of ATP, and 45% is dissipated as heat.
Oxidative phosphorylation is much more effective than anaerobic synthesis: if during the process of glycolysis, only 2 ATP molecules are synthesized during the breakdown of a glucose molecule, then 36 ATP molecules are formed during oxidative phosphorylation.
3. Photophosphorylation– the process of ATP synthesis using the energy of sunlight. This pathway of ATP synthesis is characteristic only of cells capable of photosynthesis (green plants, cyanobacteria). The energy of solar light quanta is used by photosynthetics during the light phase of photosynthesis for the synthesis of ATP.
3. Biological significance of ATP
ATP is at the center of metabolic processes in the cell, being a link between the reactions of biological synthesis and decay. The role of ATP in a cell can be compared to the role of a battery, since during the hydrolysis of ATP the energy necessary for various vital processes is released (“discharge”), and in the process of phosphorylation (“charging”) ATP again accumulates energy.

Due to the energy released during ATP hydrolysis, almost all vital processes in the cell and body occur: transmission of nerve impulses, biosynthesis of substances, muscle contractions, transport of substances, etc.
III. Consolidation of knowledge
Solving biological problems
Task 1. When we run fast, we breathe quickly, and increased sweating occurs. Explain these phenomena.
Problem 2. Why do freezing people start stamping and jumping in the cold?
Task 3. In the famous work of I. Ilf and E. Petrov “The Twelve Chairs”, among many useful tips one can find the following: “Breathe deeply, you are excited.” Try to justify this advice from the point of view of the energy processes occurring in the body.
IV. Homework
Start preparing for the test and test (dictate the test questions - see lesson 21).
Lesson 20. Generalization of knowledge in the section “Chemical organization of life”
Equipment: tables on general biology.
I. Generalization of knowledge of the section
Students work with questions (individually) followed by checking and discussion
1. Give examples of organic compounds, which include carbon, sulfur, phosphorus, nitrogen, iron, manganese.
2. How can you distinguish a living cell from a dead one based on its ionic composition?
3. What substances are found in the cell in undissolved form? What organs and tissues do they contain?
4. Give examples of macroelements included in the active sites of enzymes.
5. What hormones contain microelements?
6. What is the role of halogens in the human body?
7. How do proteins differ from artificial polymers?
8. How do peptides differ from proteins?
9. What is the name of the protein that makes up hemoglobin? How many subunits does it consist of?
10. What is ribonuclease? How many amino acids does it contain? When was it synthesized artificially?
11. Why is the rate of chemical reactions without enzymes low?
12. What substances are transported by proteins across the cell membrane?
13. How do antibodies differ from antigens? Do vaccines contain antibodies?
14. What substances do proteins break down into in the body? How much energy is released? Where and how is ammonia neutralized?
15. Give an example of peptide hormones: how are they involved in the regulation of cellular metabolism?
16. What is the structure of the sugar with which we drink tea? What three other synonyms for this substance do you know?
17. Why is the fat in milk not collected on the surface, but rather in the form of a suspension?
18. What is the mass of DNA in the nucleus of somatic and germ cells?
19. How much ATP is used by a person per day?
20. What proteins do people use to make clothes?

Primary structure of pancreatic ribonuclease (124 amino acids)
II. Homework.
Continue preparing for the test and test in the section “Chemical organization of life.”
Lesson 21. Test lesson on the section “Chemical organization of life”
I. Conducting an oral test on questions
1. Elementary composition of the cell.
2. Characteristics of organogenic elements.
3. Structure of the water molecule. Hydrogen bonding and its significance in the “chemistry” of life.
4. Properties and biological functions of water.
5. Hydrophilic and hydrophobic substances.
6. Cations and their biological significance.
7. Anions and their biological significance.
8. Polymers. Biological polymers. Differences between periodic and non-periodic polymers.
9. Properties of lipids, their biological functions.
10. Groups of carbohydrates, distinguished by structural features.
11. Biological functions of carbohydrates.
12. Elementary composition of proteins. Amino acids. Peptide formation.
13. Primary, secondary, tertiary and quaternary structures of proteins.
14. Biological function of proteins.
15. Differences between enzymes and non-biological catalysts.
16. Structure of enzymes. Coenzymes.
17. Mechanism of action of enzymes.
18. Nucleic acids. Nucleotides and their structure. Formation of polynucleotides.
19. Rules of E. Chargaff. The principle of complementarity.
20. Formation of a double-stranded DNA molecule and its spiralization.
21. Classes of cellular RNA and their functions.
22. Differences between DNA and RNA.
23. DNA replication. Transcription.
24. Structure and biological role of ATP.
25. Formation of ATP in the cell.
II. Homework
Continue preparing for the test in the section “Chemical organization of life.”
Lesson 22. Test lesson on the section “Chemical organization of life”
I. Conducting a written test
Option 1
1. There are three types of amino acids - A, B, C. How many variants of polypeptide chains consisting of five amino acids can be built. Please indicate these options. Will these polypeptides have the same properties? Why?
2. All living things consist mainly of carbon compounds, and the carbon analogue, silicon, the content of which in the earth’s crust is 300 times greater than carbon, is found only in very few organisms. Explain this fact in terms of the structure and properties of the atoms of these elements.
3. ATP molecules labeled with radioactive 32P at the last, third phosphoric acid residue were introduced into one cell, and ATP molecules labeled with 32P at the first residue closest to ribose were introduced into the other cell. After 5 minutes, the content of inorganic phosphate ion labeled with 32P was measured in both cells. Where will it be significantly higher?
4. Research has shown that 34% of the total number of nucleotides of this mRNA is guanine, 18% is uracil, 28% is cytosine and 20% is adenine. Determine the percentage composition of the nitrogenous bases of double-stranded DNA, of which the indicated mRNA is a copy.
Option 2
1. Fats constitute the “first reserve” in energy metabolism and are used when the reserve of carbohydrates is exhausted. However, in skeletal muscles, in the presence of glucose and fatty acids, the latter are used to a greater extent. Proteins are always used as a source of energy only as a last resort, when the body is starving. Explain these facts.
2. Ions of heavy metals (mercury, lead, etc.) and arsenic are easily bound by sulfide groups of proteins. Knowing the properties of sulfides of these metals, explain what will happen to the protein when combined with these metals. Why are heavy metals poisons for the body?
3. In the oxidation reaction of substance A into substance B, 60 kJ of energy is released. How many ATP molecules can be maximally synthesized in this reaction? How will the rest of the energy be used?
4. Studies have shown that 27% of the total number of nucleotides of this mRNA is guanine, 15% is uracil, 18% is cytosine and 40% is adenine. Determine the percentage composition of the nitrogenous bases of double-stranded DNA, of which the indicated mRNA is a copy.
To be continued
You understood for yourself from the previous article, because... it is very important. Now let's talk about how the movement of the myosin bridge is maintained, where the energy for contractile processes in the muscle comes from.
For our entire body ATP serves as one of the main sources of energy and muscle fiber is no exception. Let me remind you: – an intracellular source of energy that supports all processes occurring in the cell.
It is precisely the breakdown of the ATP molecule that occurs with release of energy, also during the breakdown, orthophosphoric acid is released, and ATP is converted into adenesine diphosphate (ADP).
When interacting with the actin filament, the heads of the myosin bridges split the ATP molecule, thereby obtaining energy for contraction.
However, it should be understood that the content of “spare” ATP molecules in our body is small, therefore, for long-term muscle work and, especially for intense training, our body needs energy replenishment.
Replenishment of energy resources in the muscle is carried out in three main ways:
- Breakdown of creatine phosphate. During this reaction, the creatine phosphate molecule donates its phosphate group to the adenesine diphosphate (ADP) molecule, as a result of which ADP is converted back into ATP, and creatine phosphate into creatine.
However, such energy replenishment lasts a very limited time, maintaining the energy balance of the muscles only at the very beginning of their work. This is due to the small supply of creatine phosphate in muscle cells. Next, glycolysis and oxidation in mitochondria are included in the work. - Glycolysis. During this chemical process, two molecules of lactic acid are formed in the muscle - as a result of the breakdown of a glucose molecule. The breakdown of glucose occurs with the participation of ten special enzymes.
The breakdown of one glucose molecule is capable of replenish energy reserves two ATP molecules. Glycolysis very quickly replenishes muscle ATP reserves, because occurs without the participation of oxygen (anaerobic process).
In muscle tissue, the main substrate of glycolysis is glycogen. Glycogen– a complex carbohydrate consisting of branched chain units. The bulk of carbohydrates in our body accumulates in the form of glycogen, concentrated in skeletal muscles and the liver. Glycogen reserves largely determine the volume of our muscles and the energy potential of the muscles. - Oxidation of organic substances. This process occurs with the participation of oxygen (aerobic process), and the presence of special enzymes is also necessary for its occurrence. Oxygen delivery takes a certain time, so this process starts after the breakdown of creatine phosphate and glycolysis.
The oxidation of organic substances is carried out in stages: the process of glycolysis starts, but still unformed molecules of lactic acid (pyruvate molecules) are sent to mitochondria for further oxidative processes, as a result of which energy is generated with the release of water (H2O) and carbon dioxide (CO2). With the help of the generated energy, 38 ATP molecules are formed.
If, as a result of the anaerobic breakdown of glucose (glycolysis), 2 ATP molecules are restored, then the aerobic process (oxidation in mitochondria) can restore 19 times more ATP molecules.
Conclusion: the ATP molecule is the main and universal energy source for muscle activity, but ATP reserves in muscle fiber are small, therefore they are constantly replenished by the breakdown of creatine phosphate, glycolysis and the oxidation of organic substances in mitochondria.
Moreover, glycolysis and oxidation are the main ways of ATP recovery, and each of these methods corresponds to its own type of muscle fiber. We will talk about this in the article.
The materials in this article are protected by copyright law. Copying without providing a link to the source and notifying the author is PROHIBITED!