Differing in similar properties and united by a common formula that describes the regularity of the structural difference of each subsequent member of the group from the previous one. For example, the homologous series of alkanes, or other groups. is of great importance for research, forecasting or practical application. For organic substances combined in a group, regular changes in chemical and physical properties are observed, and all of them correlate with a change in molecular weight.
Equally important are the rules that describe how the properties of substances change when moving from one group to another. To understand what a homologous series is, one should consider specific examples. For any group of compounds, increasing melting (crystallization), boiling (condensation) and density temperatures with an increase in molecular weight and the number of carbon atoms in a molecule are characteristic.
They are called saturated or paraffin; they are acyclic (there are no cycles) compounds of a normal or branched structure, the atoms in the molecules of which are connected by single bonds. The general formula has the form CnH2n+2 and describes the homologous series of alkanes. The molecule of each next member increases in comparison with the previous one by one C atom and two H atoms. K include:
- methane;
- ethane;
- propane and so on.
They also include cycloparaffins. This is a large group of organic compounds whose molecules are closed by rings. Their homologous series has the formula CnH2n, starting with a chemical with three carbon atoms. Examples of cycloparaffins:
- cyclopropane;
- cyclobutane;
- cyclopentane and so on
Unsaturated or unsaturated hydrocarbons are also acyclic. These include substances of normal and isostructure. The homologous series of alkenes has the general formula CnH2n. These compounds are distinguished by the presence of one double bond between two carbon atoms. If the previous series began with a hydrocarbon with one carbon atom (methane), then this one begins with a substance whose molecule contains two carbon atoms. Examples of alkenes:
- ethene;
- propene;
- butene and so on.
Hydrocarbons, in the molecule of which two carbon atoms are connected by a triple bond, are even more unsaturated, otherwise they are called acetylenic. They are united by the homologous series of alkynes. It is described by the formula CnH2n-2 and begins with acetylene, which has two C atoms in the formula. Examples of alkynes:
- ethyn;
- propyne;
- butin-1 and so on.
Unsaturated acyclic hydrocarbons, in the molecule of which there are two double bonds, are called dienes. They have the general formula CnH2n-2. Their homologous series starts with a hydrocarbon with three carbon atoms per molecule. Double bonds can be conjugated (separated by one single bond), cumulated (located on neighboring atoms), or isolated (separated by several single bonds). Examples of dienes:
- 1,2-propadiene;
- 1,3-butadiene;
- isoprene and so on
A special group is formed by cyclic structures, in the molecule of which there is a benzene ring. The homologous series of the simplest aromatic hydrocarbons begins with a compound with six carbon atoms - benzene. series are formed when one or more hydrogen atoms attached to the benzene ring are replaced by radicals. Thus, a number of substances are obtained: benzene, toluene, xylene. If there are two or more substituents in a molecule, then they speak of the presence of isomers for these substances. Other homologous series of aromatic compounds are formed from naphthalene, anthracene and other substances.
If there is a functional group in the hydrocarbon molecule, then such chemical compounds also form a homologous series.
- A number of alcohols are distinguished by the presence of a hydroxyl group (-OH) in the molecule. For monohydric alcohols, one hydrogen atom in the acyclic hydrocarbon is replaced by a hydroxyl group; their formula: CnH2n+1OH. There are also rows
- A number of phenols are also characterized by the presence of a hydroxyl group (-OH) in the molecule, but it replaces hydrogen in the benzene ring.
- A number of aldehydes are distinguished by the presence of a carbonyl group (>C=O) in the molecule of the chemical compound; general formula of aldehydes: R-CH=O.
- A number of ketones are also distinguished by the presence of a carbonyl group (> C \u003d O), but if in aldehydes it is connected to one radical, then in ketones there are two hydrocarbon radicals. Ketone formula: R1-CO-R2.
- A number of carboxylic acids are distinguished from other chemicals by a carboxyl group that combines carbonyl and hydroxyl groups. The formula is RCOOH.
For each series, whether it is a homologous series of aldehydes, carboxylic (organic) acids, alcohols or other substances, their properties will be largely determined by the type of functional group and will naturally change along with an increase in the molecular weight of the substance. Such a classification of a vast class of chemical compounds helps to understand nature and study their properties.
Alkanes are a class of hydrocarbons with the general formula C n H 2n+2. Related compounds that differ by one methylene group -CH 2 - form a homologous series of alkanes. The simplest substance in the series is methane with one carbon atom (CH 4).
homologues
Related compounds - homologues - are chemically similar, but have different physical properties. Depending on the number of carbon atoms, gaseous, liquid, solid alkanes are isolated. The first four representatives are gases, homologues with 5-15 carbon atoms are flammable liquids. Higher alkanes are waxes and solids with 16-390 carbon atoms.

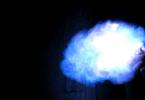
Rice. 1. Combustion of methane.
The names of alkanes are distinguished by the suffix -an after the Greek designation for the numeral:
- un- or gene- - one;
- to- - two;
- three - three;
- tetra---four;
- pent - five;
- hex - six;
- hept- - seven;
- oct - eight;
- non- - nine;
- Dec - ten.
The names of the first four homologs are fixed historically. Every tenth name "passes" to the next nine substances, retaining the numeral prefixes and the class suffix. The table of the homologous series of alkanes describes the first 20 homologues.
|
Name |
Formula |
Physical Properties |
|
Gases. They burn with a blue flame, releasing a large amount of heat. |
||
|
Flammable oily liquids. Contained in oil. Used to produce liquid fuels - gasoline, kerosene, fuel oil |
||
|
Tridecan |
||
|
Tetradecane |
||
|
Pentadecan |
||
|
Hexadecane |
Waxes and solids. Used to make vaseline, paraffin |
|
|
Heptadecane |
||
|
Octadecan |
||
|
Nanadekan |
||
The melting and boiling points of alkanes increase with the increase in the number of carbon atoms and, accordingly, the molecular weight. At the same time, all alkanes have a density less than unity. Alkanes float on the surface of water and dissolve only in organic solvents.
Isomers
Alkanes are non-cyclic saturated hydrocarbons. Molecules are long or branched carbon chains. Homologous alkanes can form isomers. The more carbon atoms, the more isomer options. The first three alkanes (methane, ethane, propane) do not form isomers. Butane, pentane, hexane have only structural isomers. Butane has two: n-butane and isobutane. Pentane forms n-pentane, isopentane, neopentane. Hexane has five isomers: n-hexane, isohexane, 3-methylpentane, diisopropyl, neohexane.
Homologues from heptane and above, in addition to structural isomers, form stereoisomers or spatial isomers that differ in the position of atoms in space. Two molecules are identical in structure and structure, but look like an object and its mirror image.

Rice. 2. Stereoisomers.
Long names of isomers are compiled according to the international IUPAC nomenclature. The verbal designation consists of three parts:
- numbers and prefixes indicating the number of affiliated groups;
- group names;
- the name of the main (longest) chain.
For example, the name of the heptane isomer, 2,3-dimethylpentane, indicates that the molecule consists of five carbon atoms (pentane) and two methyl groups attached to the second and third carbon atoms.
Structural formulas are used to display the structure of isomers. The -CH 3 methyl group is written either through a line up or down from the carbon atom, or in brackets after the -CH 2 group in the carbon chain. For example, H 3 C-CH 2 -CH(CH 2 CH 3)-CH 2 -CH 3 .

Rice. 3. Structural formula.
The number of isomers for each alkane can be calculated mathematically. Therefore, many isomers exist only in theory. It is assumed that hectane (C 100 H 202) can have 592 107 ∙ 10 34 isomers, and this is far from the last alkane in the homologous series.
What have we learned?
Alkanes are formed by the homologous series of methane with the general formula C n H 2n+2. Each subsequent homologue differs from the previous one by one CH 2 group. As carbon atoms increase in the homologous series, the physical state of substances changes. Higher alkanes are compounds containing more than 15 carbon atoms. These are solids. Liquids contain 5-15 carbon atoms, gases - 1-4. Starting from the fourth homologue, all alkanes form structural isomers. In addition, alkanes from heptane and above can form stereoisomers.
Topic quiz
Report Evaluation
Average rating: 4.2. Total ratings received: 212.
Limit (saturated) hydrocarbons called hydrocarbons, in the molecules of which the carbon atoms are interconnected by a simple bond, and all valence units not spent on the bond between the carbon atoms are saturated with hydrogen atoms.
Representatives of saturated hydrocarbons are methane CH 4 ; ethane C 2 H 6 ; propane C 3 H 8 ; butane C 4 H 10 ; pentane C 5 H 12 ; hexane C 6 H 14 . However, this series can be continued. There are carbohydrates C 30 H 62, C 50 H 102, C 70 H 142, C 100 H 202.
If we consider the hydrocarbons of the methane series, then it is easy to see that each subsequent hydrocarbon can be produced from the corresponding previous one by replacing one hydrogen atom with a CH 3 (methyl) group. Thus, the composition of the subsequent hydrocarbon molecule increases by the CH 2 group.
A number of chemical compounds of the same structural type, differing from each other by one or more structural units (usually by the CH 2 group), is called a homology series, and each of the carbohydrates – a member of a homologous series or a homologue. If we arrange the homologues in ascending order of their relative molecular weight, they form a homologous series.
The CH 2 group is called the homologous difference or the homologous difference. The general formula of saturated hydrocarbons is C n H 2 n + 2, where n – the number of carbon atoms in a molecule.
If a hydrogen atom is taken away from a hydrocarbon molecule, then the remainder of the molecule with an open bond is called a hydrocarbon radical (denoted by the letter R). Free radicals do not exist due to their high reactivity.
homology phenomenon– the existence of series of organic compounds in which the formula of any two neighbors of the series differs by the same group (most often CH 2). The physicochemical properties of the compounds change along the homologous series. In organic chemistry, the concept of homology is based on the fundamental position that the chemical and physical properties of a compound are determined by the structure of its molecules: these properties are determined by both the functional groups of the compound and its carbon skeleton.
 The whole complex of chemical properties and, consequently, the assignment of a compound to a certain class, is determined precisely by functional groups, but the degree of manifestation of chemical properties or physical properties depends on the carbon skeleton of the molecule.
The whole complex of chemical properties and, consequently, the assignment of a compound to a certain class, is determined precisely by functional groups, but the degree of manifestation of chemical properties or physical properties depends on the carbon skeleton of the molecule.
In the absence of isomerism in the case of similar carbon skeletons of compounds, the formula of homologous compounds can be written as X – (CH 2) n – Y, compounds with different numbers n of methylene units are homologues and belong to the same class of compounds. So, homologue compounds belong to the same class of compounds, and the properties of the nearest homologues are the closest.
In homologous series there is a certain regular change in properties from the younger members of the series to the older ones, but this pattern is not always observed, in some cases it may be violated. Most often this happens at the beginning of the series, because hydrogen bonds are formed in the presence of functional groups capable of forming them.
An example of a homologous series is the series of saturated hydrocarbons (alkanes). Its simplest representative – methane CH4. Methane homologues are: ethane C 2 H 6 ; propane C 3 H 8 ; butane C 4 H 10 ; pentane C 5 H 12 ; hexane C 6 H 14, heptane C 7 H 16, octane - C 8 H 18, nonane - C 9 H 20, decane - C 10 H 22, undecane - C 11 H 24, nodecane – C 12 H 26, tridecane – C 13 H 28, tetradecane – C 14 H 30, pentadecane – C 15 H 32, eicosan - C 20 H 42, pentacosan - C 25 H 52, triacontane - C 30 H 62, tetracontane - C 40 H 82, hectane - C 100 H 202.
Do you have any questions? Do you know what a homologous series is?
To get the help of a tutor - register.
The first lesson is free!
site, with full or partial copying of the material, a link to the source is required.
From Wikipedia, the free encyclopedia
 Homologous series- a number of chemical compounds of the same structural type (for example, alkanes or aliphatic alcohols - fatty alcohols), differing from each other in composition by a certain number of repeating structural units - the so-called homologous difference. Homologs- substances that belong to the same homologous series.
Homologous series- a number of chemical compounds of the same structural type (for example, alkanes or aliphatic alcohols - fatty alcohols), differing from each other in composition by a certain number of repeating structural units - the so-called homologous difference. Homologs- substances that belong to the same homologous series.
The simplest example of a homologous series is alkanes (general formula C n H 2n+2): methane CH 4, ethane C 2 H 6, propane C 3 H 8, etc.; the homological difference of this series is the methylene unit -CH 2 -.
Homology and structure of compounds
The concept of homology in organic chemistry is based on the fundamental position that the chemical and physical properties of a substance are determined by the structure of its molecules: these properties are defined as functional groups of the compound (hydroxyl alcohols, carboxyl group of carboxylic acids, aryl group of aromatic compounds, etc.) , and its carbon skeleton.
The complex of chemical properties itself and, accordingly, the belonging of a compound to a certain class, is determined precisely by functional groups (for example, the presence of a carboxyl group determines the manifestation of acidic properties by the compound and its belonging to the class of carboxylic acids), but on the degree of manifestation of chemical properties (for example, reactivity and dissociation constant) or physical properties (boiling and melting points, refractive index, etc.) also affects the carbon skeleton of the molecule (see Fig. 1).
In the case of the similarity of the carbon skeletons of the compounds, that is, the absence of isomerism, the formula of homologous compounds can be written as X-(CH 2) n-Y, compounds with different number n methylene units are homologues and belong to the same class of compounds (for example, H-(CH 2) n-COOH- aliphatic carboxylic acids). Thus, homologue compounds belong to the same class of compounds, and the properties of the nearest homologues are the closest.
In the homologous series, there is a regular change in properties from the younger members of the series to the older ones, however, this pattern can be violated, first of all, at the beginning of the series, due to the formation of hydrogen bonds in the presence of functional groups capable of their formation (see Fig. 2, melting point).
In the study of parallelisms in the phenomena of hereditary variability, N. I. Vavilov, by analogy with the homologous series of organic compounds, introduced the concept Homologous series in hereditary variability.
see also
Write a review on the article "Homological series"
An excerpt characterizing the Homological series
After Prince Andrei, Boris approached Natasha, inviting her to dance, and that adjutant dancer who started the ball, and still young people, and Natasha, passing her excess gentlemen to Sonya, happy and flushed, did not stop dancing the whole evening. She did not notice and did not see anything that occupied everyone at this ball. She not only did not notice how the sovereign spoke for a long time with the French envoy, how he spoke especially graciously with such and such a lady, how the prince did such and such and said how Helen had great success and received special attention such and such; she did not even see the sovereign and noticed that he left only because after his departure the ball became more lively. One of the merry cotillions, before supper, Prince Andrei again danced with Natasha. He reminded her of their first meeting in Otradnenskaya Alley and how she could not fall asleep on a moonlit night, and how he could not help hearing her. Natasha blushed at this reminder and tried to justify herself, as if there was something shameful in the feeling in which Prince Andrei involuntarily overheard her.
Prince Andrei, like all people who grew up in the world, loved to meet in the world that which did not have a common secular imprint. And such was Natasha, with her surprise, joy and timidity, and even mistakes in French. He spoke with her especially tenderly and carefully. Sitting beside her, talking to her about the simplest and most insignificant subjects, Prince Andrei admired the joyful gleam in her eyes and smile, which related not to spoken speeches, but to her inner happiness. While Natasha was chosen and she got up with a smile and danced around the hall, Prince Andrei admired in particular her timid grace. In the middle of the cotillion, Natasha, having finished the figure, still breathing heavily, approached her place. The new gentleman again invited her. She was tired and out of breath, and apparently thought of refusing, but immediately again cheerfully raised her hand on the cavalier's shoulder and smiled at Prince Andrei.
“I would be glad to rest and sit with you, I am tired; but you see how they choose me, and I'm glad about it, and I'm happy, and I love everyone, and you and I understand all this, ”and that smile said a lot more. When the gentleman left her, Natasha ran across the hall to take two ladies for the pieces.
“If she comes first to her cousin, and then to another lady, then she will be my wife,” Prince Andrei said quite unexpectedly to himself, looking at her. She went first to her cousin.
“What nonsense sometimes comes to mind! thought Prince Andrei; but it’s only true that this girl is so sweet, so special, that she won’t dance here for a month and get married ... This is a rarity here, ”he thought, when Natasha, straightening the rose that had fallen back from her corsage, sat down beside him.
At the end of the cotillion, the old count in his blue tailcoat approached the dancers. He invited Prince Andrei to his place and asked his daughter if she was having fun? Natasha did not answer and only smiled with such a smile that said reproachfully: "How could you ask about this?"
- So much fun, like never before in my life! - she said, and Prince Andrei noticed how quickly her thin hands rose to hug her father and immediately fell. Natasha was as happy as ever in her life. She was at that highest stage of happiness when a person becomes completely trusting and does not believe in the possibility of evil, misfortune and grief.
Pierre at this ball for the first time felt insulted by the position that his wife occupied in higher spheres. He was sullen and distracted. There was a wide crease across his forehead, and he, standing at the window, looked through his glasses, seeing no one.
Natasha, on her way to dinner, walked past him.
The gloomy, unhappy face of Pierre struck her. She stopped in front of him. She wanted to help him, to convey to him the surplus of her happiness.
“How fun, Count,” she said, “isn't it?
Pierre smiled absently, obviously not understanding what was being said to him.
“Yes, I am very glad,” he said.
“How can they be dissatisfied with something,” thought Natasha. Especially one as good as this Bezukhov?” In Natasha's eyes, all those who were at the ball were equally kind, sweet, wonderful people who loved each other: no one could offend each other, and therefore everyone should have been happy.
The next day, Prince Andrei remembered yesterday's ball, but did not dwell on it for a long time. “Yes, the ball was very brilliant. And yet ... yes, Rostova is very nice. There is something fresh, special, not Petersburg, which distinguishes her. That's all he thought about yesterday's ball, and after drinking tea, he sat down to work.
But from fatigue or insomnia (the day was not good for classes, and Prince Andrei could not do anything), he criticized his work himself, as often happened to him, and was glad when he heard that someone had arrived.
The visitor was Bitsky, who served in various commissions, visited all the societies of St. Petersburg, a passionate admirer of new ideas and Speransky, and an anxious news reporter of St. Petersburg, one of those people who choose a trend like a dress - according to fashion, but who for this reason seem to be the most ardent partisans of trends . He anxiously, barely having time to take off his hat, ran to Prince Andrei and immediately began to speak. He had just learned the details of the meeting of the State Council this morning, opened by the sovereign, and enthusiastically talked about it. The emperor's speech was extraordinary. It was one of those speeches only given by constitutional monarchs. “The sovereign directly said that the council and the senate are state estates; he said that government should not be based on arbitrariness, but on firm principles. The sovereign said that the finances should be transformed and the reports should be made public,” Bitsky said, hitting on well-known words and opening his eyes significantly.
Limit (saturated) hydrocarbons called hydrocarbons, in the molecules of which the carbon atoms are interconnected by a simple bond, and all valence units not spent on the bond between the carbon atoms are saturated with hydrogen atoms.
Representatives of saturated hydrocarbons are methane CH 4 ; ethane C 2 H 6 ; propane C 3 H 8 ; butane C 4 H 10 ; pentane C 5 H 12 ; hexane C 6 H 14 . However, this series can be continued. There are carbohydrates C 30 H 62, C 50 H 102, C 70 H 142, C 100 H 202.
If we consider the hydrocarbons of the methane series, then it is easy to see that each subsequent hydrocarbon can be produced from the corresponding previous one by replacing one hydrogen atom with a CH 3 (methyl) group. Thus, the composition of the subsequent hydrocarbon molecule increases by the CH 2 group.
A number of chemical compounds of the same structural type, differing from each other by one or more structural units (usually by the CH 2 group), is called a homology series, and each of the carbohydrates – a member of a homologous series or a homologue. If we arrange the homologues in ascending order of their relative molecular weight, they form a homologous series.
The CH 2 group is called the homologous difference or the homologous difference. The general formula of saturated hydrocarbons is C n H 2 n + 2, where n – the number of carbon atoms in a molecule.
If a hydrogen atom is taken away from a hydrocarbon molecule, then the remainder of the molecule with an open bond is called a hydrocarbon radical (denoted by the letter R). Free radicals do not exist due to their high reactivity.
homology phenomenon– the existence of series of organic compounds in which the formula of any two neighbors of the series differs by the same group (most often CH 2). The physicochemical properties of the compounds change along the homologous series. In organic chemistry, the concept of homology is based on the fundamental position that the chemical and physical properties of a compound are determined by the structure of its molecules: these properties are determined by both the functional groups of the compound and its carbon skeleton.
 The whole complex of chemical properties and, consequently, the assignment of a compound to a certain class, is determined precisely by functional groups, but the degree of manifestation of chemical properties or physical properties depends on the carbon skeleton of the molecule.
The whole complex of chemical properties and, consequently, the assignment of a compound to a certain class, is determined precisely by functional groups, but the degree of manifestation of chemical properties or physical properties depends on the carbon skeleton of the molecule.
In the absence of isomerism in the case of similar carbon skeletons of compounds, the formula of homologous compounds can be written as X – (CH 2) n – Y, compounds with different numbers n of methylene units are homologues and belong to the same class of compounds. So, homologue compounds belong to the same class of compounds, and the properties of the nearest homologues are the closest.
In homologous series there is a certain regular change in properties from the younger members of the series to the older ones, but this pattern is not always observed, in some cases it may be violated. Most often this happens at the beginning of the series, because hydrogen bonds are formed in the presence of functional groups capable of forming them.
An example of a homologous series is the series of saturated hydrocarbons (alkanes). Its simplest representative – methane CH4. Methane homologues are: ethane C 2 H 6 ; propane C 3 H 8 ; butane C 4 H 10 ; pentane C 5 H 12 ; hexane C 6 H 14, heptane C 7 H 16, octane - C 8 H 18, nonane - C 9 H 20, decane - C 10 H 22, undecane - C 11 H 24, nodecane – C 12 H 26, tridecane – C 13 H 28, tetradecane – C 14 H 30, pentadecane – C 15 H 32, eicosan - C 20 H 42, pentacosan - C 25 H 52, triacontane - C 30 H 62, tetracontane - C 40 H 82, hectane - C 100 H 202.
Do you have any questions? Do you know what a homologous series is?
To get help from a tutor -.
The first lesson is free!
blog.site, with full or partial copying of the material, a link to the source is required.